Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(T1330-08-50) Tailoring Drug Release from Long-Acting Contraceptive Levonorgestrel-Containing Intrauterine Systems

Suraj Fanse, MS (he/him/his)
PhD Graduate Student
University of Connecticut
Storrs Mansfield, Connecticut, United States
Suraj Fanse, MS (he/him/his)
PhD Graduate Student
University of Connecticut
Storrs Mansfield, Connecticut, United States- QB
Quanying Bao, Ph.D. (she/her/hers)
University of Connecticut
Storrs, Connecticut, United States - YZ
Yuan Zou, Ph.D. (he/him/his)
US Food and Drug Administration
Silverspring, Maryland, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States 
Diane J. Burgess, Ph.D. (she/her/hers)
Distinguished Professor
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The past few years have witnessed a change in women’s contraceptive choices with an increasing number of women using hormonal intrauterine systems (IUSs) to provide long-term contraception (3-8 years)1. Despite the first levonorgestrel (LNG) IUS (Mirena®) being introduced in 2000, currently, there are only four LNG-IUS products approved by the U.S. Food and Drug Administration (FDA) and there are no LNG-IUS generic products. The difficulty in developing generic LNG-IUSs stems from their ultra-long-acting nature, structural complexity (monolithic matrix-reservoir type drug-device combination), manufacturing challenges, lack of a clear understanding of excipient characteristics, locally-acting nature, and an incomplete understanding of drug release mechanisms2. The dearth of generic LNG-IUS limits access to affordable LNG-IUSs. The objectives of the present research were: (i) to identify and investigate the impact of critical material attributes and processing parameters; (ii) to investigate the role of excipients on product performance; and (iii) to elucidate drug release mechanisms of LNG-IUSs, in order to improve product understanding and enable the rational development of their generics.
Methods: IUSs with a drug loading of 50% w/w levonorgestrel (LNG) were prepared by mixing the drug and the polydimethylsiloxane (PDMS) prepolymers (elastomer base and crosslinking agent) using a planetary mixer and subsequent processing via extrusion to form cylindrical implants3. The extruded implants were cured at elevated temperatures and underwent in situ polymer crosslinking to form the matrix and then covered with an outer PDMS membrane to realize the final product (core-shell implant) (Fig.1A). Manufacturing variables (mixing methods, polymer curing temperature and time) and material attributes (polymer molecular weight, polymer chemistries, additives, and fillers) were investigated. Investigation of real-time in vitro drug release was conducted. The microstructure and molecular properties of the formulations were characterized via DSC, XRD, SEM, 13C solid-state NMR, TGA, ultra-performance liquid chromatography (UPLC) and dynamic mechanical analysis (DMA).
Results: Processing conditions such as curing temperature and time significantly affected LNG-IUS product performance. Rapid curing (e.g., 150°C for 30 min) results in partial polymer crosslinking. Such formulations with low crosslinking densities had low crystallinity and possibly non-uniform spatial drug distribution (due to inhomogeneities in crosslinking). Consequently, rapid curing led to slow drug release rates (Fig.1C). On the contrary, a slow curing rate (e.g., 80°C for 24 h) allows sufficient time for the polymer chains to orient and crosslink with each other completely (Fig.1D,F). Such formulations showed high drug release rates. The temperature dependence of crosslinking density (and thus drug release) was a function of drug loading in LNG-IUSs (Fig.1E). Different drug-polymer mixing methods (planetary mixing vs hand mixing) did not show differences in release profiles (Fig.1B). The influence of various excipients in LNG-IUSs on their performance was determined and release mechanisms were investigated. Additives that increased matrix hydrophobicity (e.g., resins) showed fast drug release rates (Fig.2A). These hydrophobic excipients facilitate the dissolution of the lipophilic drug (LNG) and increase drug permeability through the matrix. Moreover, the addition of different excipients also altered the crystallization kinetics of the crosslinked polymer. Higher amorphous domains of PDMS (low %crystallinity) contributed to higher drug solubilization in the matrix. Addition of silica (filler) reduced the release rate owing to polymer interaction via hydrogen bonding. At very low viscosities (achieved by incorporating up to 10% w/w silicone oil) only a slight increase in drug release was observed. Addition of pore-formers (10% w/w PEG) and osmotic agents (10% w/w NaCl) to the formulations did not cause a significant increase in release rates indicating that drug release is not dictated by porosity or by osmotic pressure (Fig.2E-F). Furthermore, these results implied that the extremely hydrophobic nature of PDMS in the outer membrane (sheath) prevents water absorption into the formulation. Effect of different polymer chemistries on drug release revealed the following rank order: phenyl-substituted PDMS (hydrophobic and amorphous) > dimethyl-substituted PDMS (intermediate hydrophobicity and crystalline) > trifluoropropyl-substituted PDMS (hydrophilic and amorphous). The overall drug permeability (release) was dictated by a balance between drug solubility in the polymer matrix (partition) and drug diffusivity (Fig.2G-I). Different model compounds with various physicochemical properties were used to establish a correlation between the first-order release rate constants and both the drug solubility in the polymer and the Log P (Fig.3). Overall, based on the release mechanisms explained in this study, factors controlling matrix hydrophobicity and drug-excipient interactions can be leveraged to tune LNG-IUS release rates to the target daily drug release rates in vivo.
Conclusion: Elucidating the material-property-processing relationship of LNG-IUSs will guide: (a) the rational selection of excipients; and (b) the optimum manufacturing design space. Ultimately, this will allow tailoring of drug release to establish bioequivalence with commercial reference listed drugs (RLDs) to facilitate the development of generic IUSs and improve women’s health. Furthermore, insights obtained from the current study will be beneficial to the development of other PDMS-based controlled-release products.
References: 1. Intrauterine devices (IUDs): access for women in the US. Women’s health policy. https://www.kff.org/womens-health-policy/fact-sheet/intrauterine-devices-iuds-access-for-women-in-the-u-s/, 2020.
2. Bao Q., et al. Drug release testing of long-acting intrauterine systems. J. Controlled Release, 2019. 316, 349-358.
3. Fanse S., et al. Impact of polymer crosslinking on release mechanisms from long-acting levonorgestrel intrauterine systems. Int. J. Pharm., 2022. 612, 121383.
Acknowledgements: Funding for this project was made possible by a U.S. Food and Drug Administration grant (1U01FD005443-01). This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
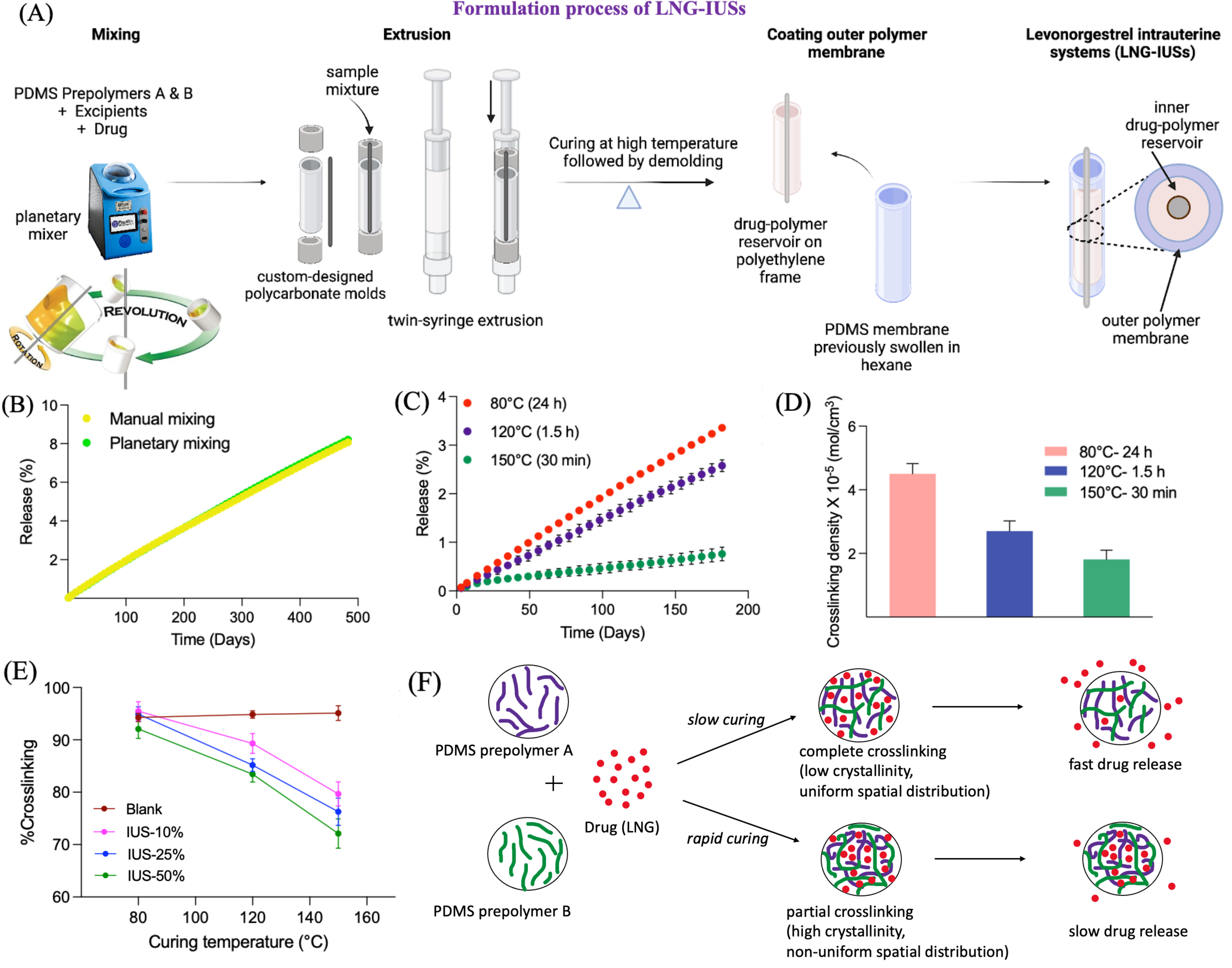 Fig. 1. A) Schematic of the formulation process of LNG-IUSs; B) effect of mixing methods (planetary mixing vs manual mixing) on drug release from LNG-IUSs (mean±SD, n=3); C) effect of curing temperature on drug release from LNG-IUSs (mean±SD, n=3); D) crosslinking density (mol/cm3) of LNG-IUSs prepared at different curing conditions (mean±SD, n=3); E) crosslinking densities (%) of LNG-IUSs with various drug loadings (10% w/w, 25% w/w, and 50% w/w LNG) processed at different curing temperatures; and F) schematic depicting the impact of curing rates on LNG-IUS performance.
Fig. 1. A) Schematic of the formulation process of LNG-IUSs; B) effect of mixing methods (planetary mixing vs manual mixing) on drug release from LNG-IUSs (mean±SD, n=3); C) effect of curing temperature on drug release from LNG-IUSs (mean±SD, n=3); D) crosslinking density (mol/cm3) of LNG-IUSs prepared at different curing conditions (mean±SD, n=3); E) crosslinking densities (%) of LNG-IUSs with various drug loadings (10% w/w, 25% w/w, and 50% w/w LNG) processed at different curing temperatures; and F) schematic depicting the impact of curing rates on LNG-IUS performance.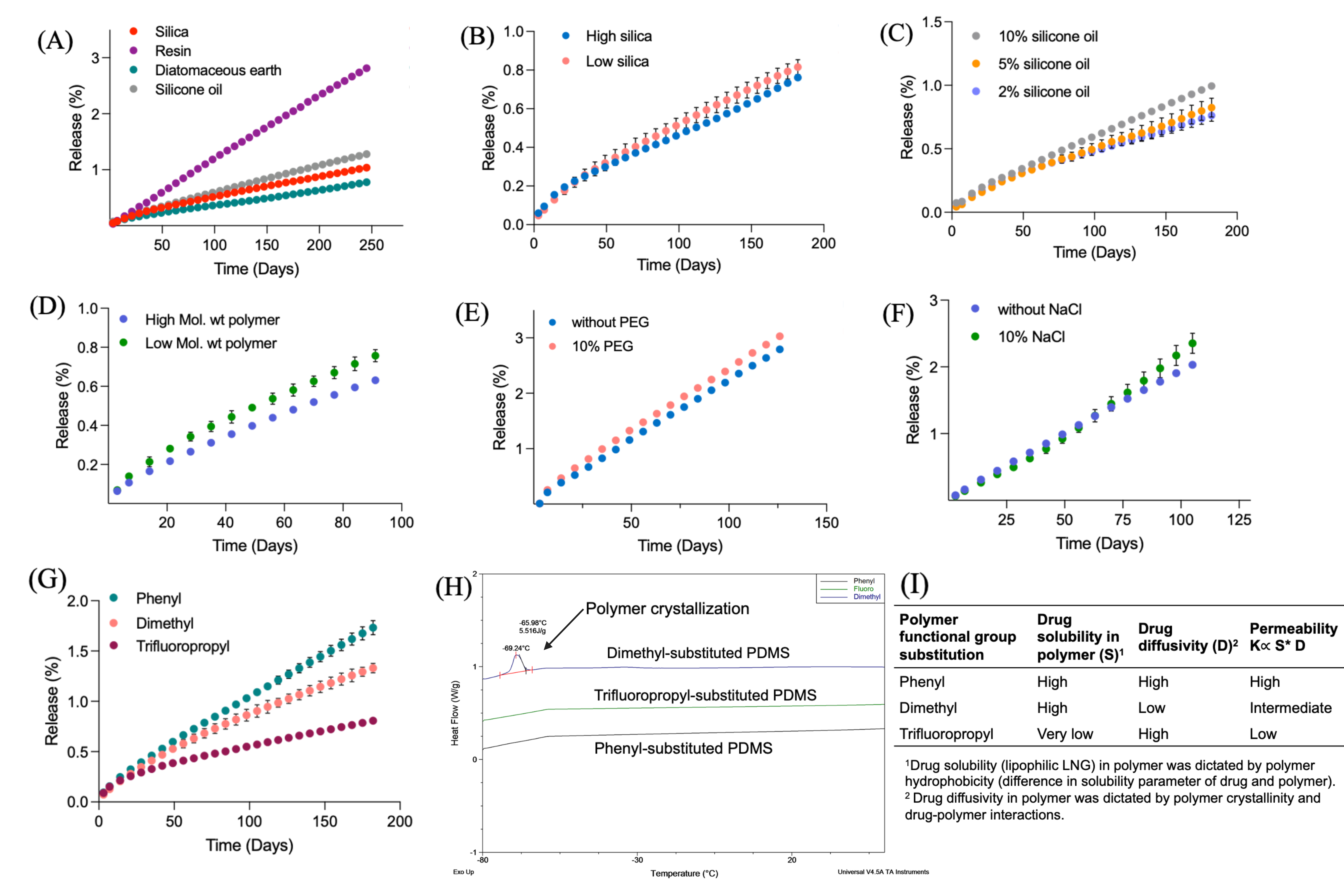 Fig. 2. Influence of various excipients to tune LNG-IUS release profiles: A) in vitro drug release profiles of formulations incorporated with different fillers (mean±SD, n=3); B) in vitro drug release profiles of formulations with varying amounts of silica (difference in silica content between both formulations was approximately 10% w/w) (mean±SD, n=3); C) in vitro drug release profiles of formulations prepared with different concentrations of silicone oil (lubricant, 350cST) (mean±SD, n=3); D) in vitro drug release profiles of formulations prepared with PDMS of different molecular weights (mean±SD, n=3); E) in vitro drug release profiles of formulations prepared without polyethylene glycol and with polyethylene glycol (pore-former) (10% w/w) (mean±SD, n=3); F) in vitro drug release profiles of formulations prepared without sodium chloride and with sodium chloride (osmotic agent) (10% w/w) (mean±SD, n=3); G) in vitro drug release profiles of formulations prepared using customized PDMS with different chemical substitutions (mean±SD, n=3); H) representative DSC thermograms of formulations prepared using customized PDMS with different chemical substitutions (exothermic peak indicates polymer crystallization); and I) summary of critical attributes governing the mass transport of drug through PDMS (the proposed mechanism of polymer-controlled release involves drug solubilization within the polymer and subsequent diffusion through the matrix and the outer polymer membrane).
Fig. 2. Influence of various excipients to tune LNG-IUS release profiles: A) in vitro drug release profiles of formulations incorporated with different fillers (mean±SD, n=3); B) in vitro drug release profiles of formulations with varying amounts of silica (difference in silica content between both formulations was approximately 10% w/w) (mean±SD, n=3); C) in vitro drug release profiles of formulations prepared with different concentrations of silicone oil (lubricant, 350cST) (mean±SD, n=3); D) in vitro drug release profiles of formulations prepared with PDMS of different molecular weights (mean±SD, n=3); E) in vitro drug release profiles of formulations prepared without polyethylene glycol and with polyethylene glycol (pore-former) (10% w/w) (mean±SD, n=3); F) in vitro drug release profiles of formulations prepared without sodium chloride and with sodium chloride (osmotic agent) (10% w/w) (mean±SD, n=3); G) in vitro drug release profiles of formulations prepared using customized PDMS with different chemical substitutions (mean±SD, n=3); H) representative DSC thermograms of formulations prepared using customized PDMS with different chemical substitutions (exothermic peak indicates polymer crystallization); and I) summary of critical attributes governing the mass transport of drug through PDMS (the proposed mechanism of polymer-controlled release involves drug solubilization within the polymer and subsequent diffusion through the matrix and the outer polymer membrane). 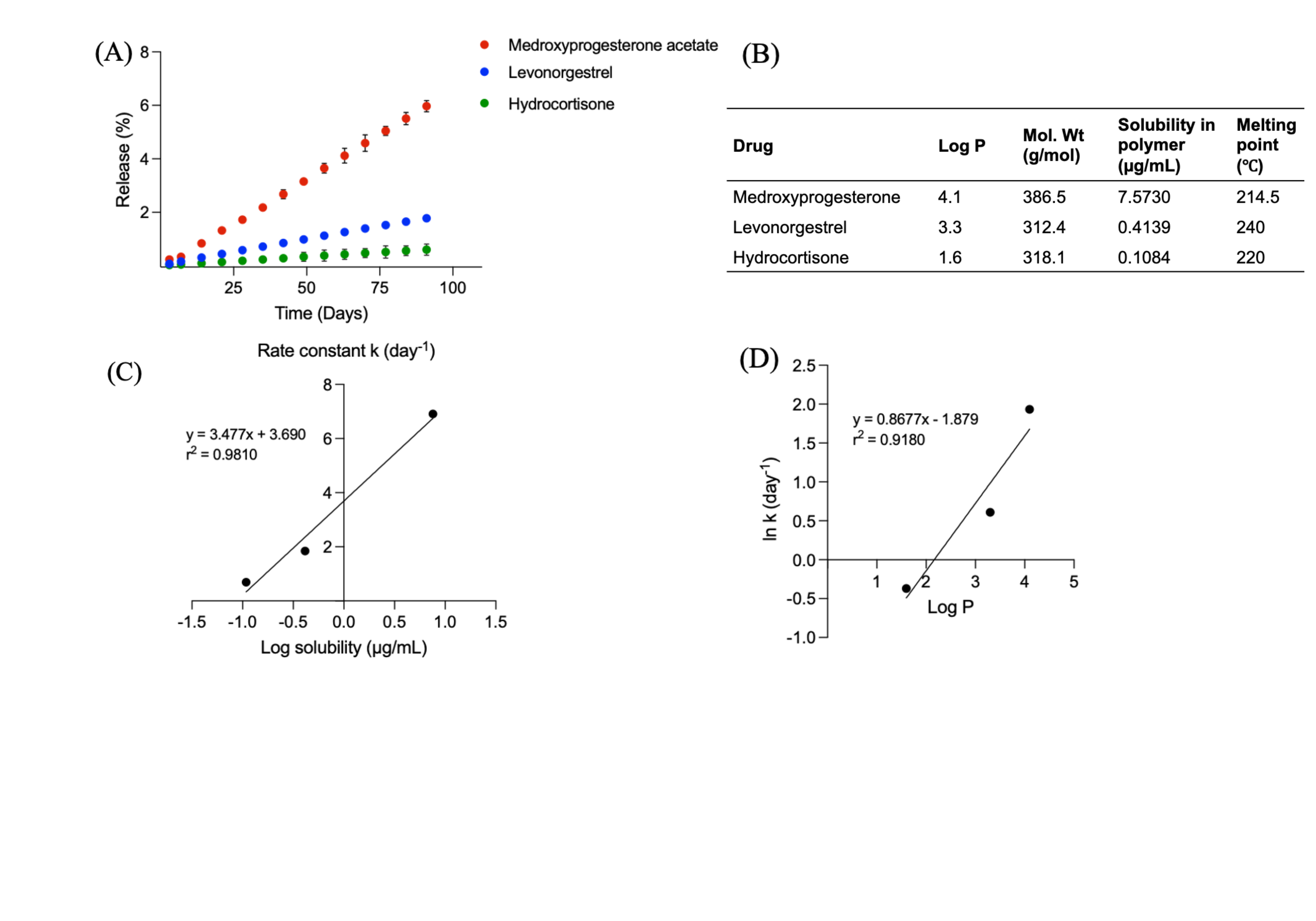 Fig. 3. A) In vitro drug release profiles of IUSs with different model compounds (mean±SD, n=3); B) physicochemical properties of different model compounds; C) correlation between first order kinetic release rate constants (obtained by model fitting of release profiles) and apparent solubility of model compounds in the polymer; and D) correlation between first order kinetic release rate constants and log P of the model compounds.
Fig. 3. A) In vitro drug release profiles of IUSs with different model compounds (mean±SD, n=3); B) physicochemical properties of different model compounds; C) correlation between first order kinetic release rate constants (obtained by model fitting of release profiles) and apparent solubility of model compounds in the polymer; and D) correlation between first order kinetic release rate constants and log P of the model compounds.