Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(M1530-12-77) Poloxamer 188 in Amorphous Solid Dispersions of Efavirenz and HPMC-AS Improves In Vitro Dissolution of the Drug but Does Not Affect In Vitro Permeation nor In Vivo Absorption
- AM
Anette Müllertz, Ph.D. (she/her/hers)
University of Copenhagen
Copenhagen, Hovedstaden, Denmark 
Mette Klitgaard, Ph.D. (she/her/hers)
Postdoc
University of Copenhagen
Copenhagen, Hovedstaden, Denmark- JJ
Jacob Rune Jørgensen, Ph.D. (he/him/his)
Bioneer A/S
Copenhagen, Hovedstaden, Denmark - WM
Wolfgang Mohr, Ph.D.
Losan Pharma GmbH
Neuenburg, Baden-Wurttemberg, Germany 
Matthias Rischer, Ph.D.
Director Drug Delivery & Innovation Projects
Losan Pharma
Neuenburg, Baden-Wurttemberg, Germany- AS
Andreas Sauer, Ph.D. (he/him/his)
SE Tylose GmbH & Co. KG
Wiesbaden, Hessen, Germany - SM
Shilpa Mistry, Ph.D.
Harke Pharma
Urmston, Manchester, England, United Kingdom - TR
Thomas Rades, Prof. Dr. Dr. h.c. (he/him/his)
University of Copenhagen
Copenhagen, Hovedstaden, Denmark
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Amorphous solid dispersions (ASDs) are a commonly applied formulation strategy to improve the oral bioavailability of poorly water-soluble drugs. However, the in vitro assessment of ASDs often fails to predict how these formulations will perform in vivo after oral administration1. A recent study obtained a linear rank order relation between AUC from an oral pharmacokinetic (PK) study of four ASDs and crystalline efavirenz (EFV) in rats and the in vitro permeation from a modified dissolution-permeation (D/P) setup using a hydrophilic filter on top of the parallel artificial membrane permeability assay (PAMPA) membrane2. The current study aimed to challenge the predictability of this modified D/P setup by testing ASDs with surfactants and evaluating whether a rank order correlation could be obtained with these more complex formulations.
Methods: Formulation preparation: EFV (20 % (w/w)) and hypromellose acetate succinate grade AS-MF were formulated into a binary ASD (MF0) and ternary ASD by adding 10 % (w/w) poloxamer 188 (MFPX10). The ASDs were prepared by vacuum compression molding into 200 mg discs (diameter 20 mm), crushed and size fractioned (180 – 355 µm) and lastly filled into size 9 rat gelatin capsules. The amorphicity of the ASDs was confirmed using X-ray powder diffraction. In vitro evaluation of the formulations: The ASDs were tested in a D/P µFLUX setup equipped with a PAMPA membrane (regular setup) and an additional hydrophilic membrane for the modified setup. The donor medium pH was 3.5 for the first 30 min, after which it was adjusted to pH 7.0 for the remaining 3.5 h. The receiver compartment contained absorption sink buffer at pH 7.4. Three non-ASD formulations were additionally tested in the setup, i.e., crystalline EFV (EFVc), amorphous EFV (EFVa), and a physical mix of EFV and the surfactant poloxamer 188 (2:1 % (w/w)) (EFV:PX). In vivo PK study: MF0, MFPX10, EFV:PX, EFVc, and EFVa were dosed with oral gavage to fasted male Sprague-Dawley rats. Blood was sampled from the tail and EFV was quantified using high-performance liquid chromatography with UV detection at 248 nm.
Results: In vitro evaluations of formulations: The addition of the polymeric surfactant in the ternary ASD (MFPX10) improved the in vitro dissolution of EFV when compared to the binary MF0 (Figure 1). When utilizing the regular PAMPA membrane setup, the permeated amount of EFV significantly increased for the MFPX10 when compared to MF0 without surfactants (p < 0.05). However, in the presence of the additional hydrophilic filter in the modified D/P setup, there was no difference in the permeated amount of EFV or flux between MFPX10 and MF0. In the regular setup, EFVc and EFV:PX resulted in a similar amount of permeated EFV as the MF0. The modified setup caused a decrease in the permeated amounts of EFV from the EFVc and EFV:PX, both performing worse than the MF0 and EFV:PX performing better than EFVc. In both setups, the EFVa performed worst in both dissolution and permeation, likely due to almost immediate precipitation and recrystallization of the amorphous drug when exposed to the donor medium. PK study and rank order prediction: The binary ASD MF0 and the ternary MFPX10 performed equally in the PK study and had a significantly improved absorption when compared to the three non-ASD formulations (p < 0.05) (Figure 2). EFV:PX trended towards higher absorption than EFVa and EFVc, and EFVa performed slightly better than EFVc although the AUC0-8h of the three non-ASDs were not significantly different. No correlation was obtained when comparing the in vivo AUC0-8h to the in vitro AUC0-4h from the regular D/P setup, whereas a linear relation of R2 0.86 was obtained from the modified D/P setup (Figure 3). Both in vitro D/P setups underestimated the oral EFVa performance likely due to overestimating the precipitation in the in vitro donor medium which contained no bile salts and lipids otherwise present in the intestinal fluids. Removing the EFVa from the comparison improved the linear rank ordering from the modified D/P setup to R2 0.93.
Conclusion: The addition of the polymeric surfactant Poloxamer 188 to the ASDs improved the dissolution but did not affect the permeation. This was evident in the modified D/P setup as well as in vivo and thus the modified D/P setup proved predictive even when challenged with more complex formulations.
References: 1 Schittny, A. et al. Drug Deliv. 27, 110–127 (2020)
2 Jørgensen, J. R. et al. Eur. J. Pharm. Biopharm. 188, 26–32 (2023)
Acknowledgements:The animal study was conducted at the University of Copenhagen (license number 2019-15-0201-00262) in accordance to the Danish law on animal experiments and the EU Directive 2010/63/EU.
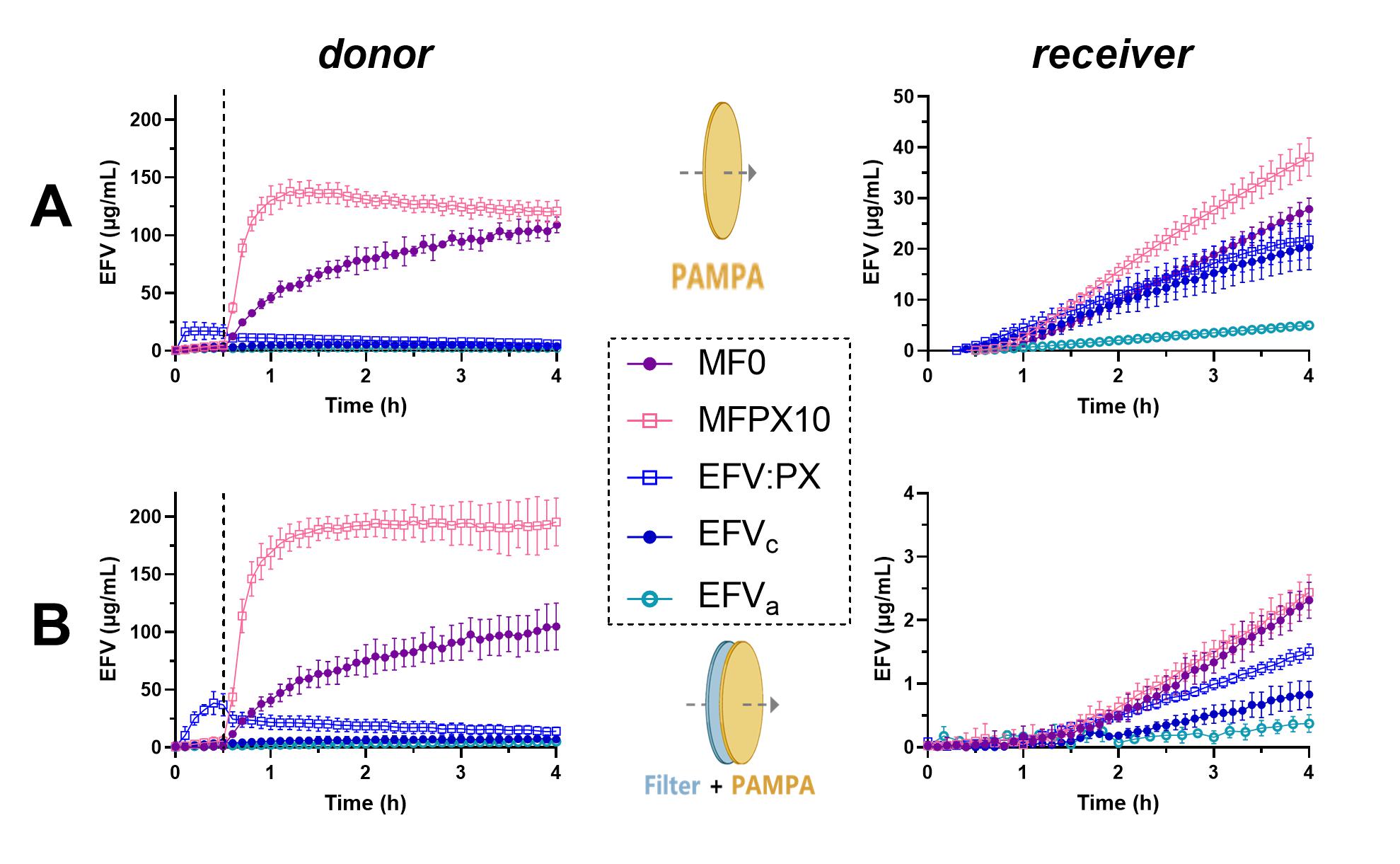 Figure 1. EFV concentrations in the donor and receiver compartments of the regular (A) and modified (B) D/P setup. The dotted line in the donor compartment shows the change from gastric pH 3.5 to intestinal pH 7.0. Data is represented as mean ±SD (n=3).
Figure 1. EFV concentrations in the donor and receiver compartments of the regular (A) and modified (B) D/P setup. The dotted line in the donor compartment shows the change from gastric pH 3.5 to intestinal pH 7.0. Data is represented as mean ±SD (n=3)..jpg) Figure 2. Pharmacokinetic profiles of EFV after oral administration of the five test formulations with equal EFV dose. Data represented as mean ± standard error of the mean (SEM) (n=5-6).
Figure 2. Pharmacokinetic profiles of EFV after oral administration of the five test formulations with equal EFV dose. Data represented as mean ± standard error of the mean (SEM) (n=5-6)..jpg) Figure 3. In vivo-in vitro relations between the in vivo AUC0-8h and the in vitro AUC0-4h from the receiver compartment in the regular (A) and modified (B) D/P setup.
Figure 3. In vivo-in vitro relations between the in vivo AUC0-8h and the in vitro AUC0-4h from the receiver compartment in the regular (A) and modified (B) D/P setup.