Formulation and Delivery - Chemical
Category: Poster Abstract
(M1530-11-72) Enhanced Dissolution of Amphotericin B through Development of Amorphous Solid Dispersions Containing Polymer and Surfactants
Monday, October 23, 2023
3:30 PM - 4:30 PM ET

Mikayla M. Smith, MS (she/her/hers)
Graduate Research Assistant
University of Kansas
Lawrence, Kansas, United States
Mikayla M. Smith, MS (she/her/hers)
Graduate Research Assistant
University of Kansas
Lawrence, Kansas, United States- TD
Tahnee J. Dening, Ph.D.
Genentech, Inc.
South San Francisco, California, United States - AB
Anil K. Basra, Ph.D.
Amgen
Thousand Oaks, California, United States - MH
Michael J. Hageman, Ph.D.
University of Kansas
Lawrence, Kansas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Amphotericin B (AmB) is the gold standard for antifungal therapy; however, its poor solubility limits its administration to intravenous infusion. A promising formulation strategy to achieve an oral formulation for AmB is the development of amorphous solid dispersions (ASDs) via spray-drying. ASDs incorporate amorphous drug within a polymeric matrix to stabilize the amorphous form. Inclusion of surfactants into ASDs is a newer concept yet offers increased dissolution opportunities when combined with a polymer. We developed both binary ASDs (AmB:polymer or AmB:surfactant) and ternary ASDs (AmB:polymer:surfactant) using a variety of surfactants to determine the optimal carbon chain length and functional group in a surfactant for achieving maximal AmB concentration in solution.
Methods: The extremely low solubility of AmB in all organic solvents, except for dimethyl sulfoxide (DMSO), required the use of liquid antisolvent precipitation (LAP) to be able to spray dry AmB. This is because the high boiling point of DMSO is not suitable for spray-drying. Using the LAP method, AmB was dissolved in DMSO and spiked into the antisolvent (ethanol). After centrifugation, the supernatant was decanted and the remaining AmB pellet was redispersed in methanol, from which AmB could be adequately spray dried. Powder X-Ray diffraction (PXRD) was used to verify the amorphous nature of the formulations. To assess the dissolution concentration of AmB in 50 mM pH 6.8 phosphate buffer, samples were taken at timepoints up to 120 minutes and analyzed using HPLC. Finally, dynamic light scattering (DLS) was used to support the presence or absence of potential interactions between the excipients or between the excipients and AmB.
Results: PXRD data confirmed the amorphous nature of the AmB ASD formulations. From the 13 different AmB ASD formulations created, three main variables were assessed: (1) binary vs. ternary; (2) carbon chain length of the surfactant; (3) charge of the functional group on the surfactant. The binary formulation containing AmB:12S (25:75) was only able to increase the dissolved concentration of AmB approximately 40-fold, while the ternary formulation containing AmB:12S:HPMCAS 912 (25:10:65) was able to increase the concentration by 90-fold. This significant difference conveys the importance of including both a surfactant and polymer to achieve maximal performance. It was hypothesized that the best surfactant for inclusion into the ASD would be one which contained the same carbon chain length as AmB (14 carbons). This was found to be true; however, there was no significant difference observed between all three surfactants which contained 14 ± 2 carbons. When comparing the AmB:12S:HPMCAS 912 (25:10:65) and the AmB:C12:HPMCAS 912 (25:10:65) formulations, the formulation containing the sulfate functional group (12S) was able to improve the concentration of AmB up to approximately 90 μg/mL whereas the carboxylate functional group (C12) only improved AmB concentration up to approximately 10 μg/mL. This is likely due to the potential for the stronger anionic group (sulfate) to interact better with the cationic amine on the polar mycosamine sugar of AmB. The significant difference in AmB concentration observed between these two formulations supports the possibility that there is an interaction occurring between the surfactant and AmB. These results, combined with the difference observed between the binary and ternary formulations, lead us to explore the possibility of excipient-drug interactions using DLS. The binary AmB:12S (25:75) formulation provided a single peak with mean diameter of 508 nm, while the ternary AmB:12S:HPMCAS 912 (25:10:65) formulation also had only a single peak with mean diameter of 776 nm. Additionally, the mean diameter of 12S:HPMCAS 912 was 587 nm. The presence of only one peak for both the binary and ternary formulations indicates that there is only one species present in the solution. This supports the idea that the excipients included in the ASD formulation are directly interacting with AmB, allowing for a higher concentration of AmB in solution.
Conclusion: A surfactant with a sulfate functional group and a carbon chain length of 14 ± 2 carbons is optimal for increasing the dissolution concentration of AmB. Additionally, there may be an interaction between the surfactant 12S and polymer HPMCAS 912 with AmB, due to the significant increase in AmB concentration when using the ternary formulation, which contains both excipients. The possibility for this interaction is further supported by the observance of a single large particle size in the DLS data as opposed to 2 or 3 separate peaks. The resulting 90-fold increase in AmB concentration from the ternary formulation could thus be occurring due to the presence of surfactant:polymer clusters, which are better able to both maintain the amorphous form of AmB and create a reserve of AmB so that a much higher concentration of AmB is available for absorption.1 Alternatively, both surfactant and polymer could be interacting separately with AmB, which would prevent self-aggregation of AmB and allow for a higher concentration of AmB in solution.
References: 1. Qi S,, Roser S, Edler K, Pigliacelli C, Rogerson M, Weuts I, Van Dycke F, Stokbroekx S. Insights into the Role of Polymer-Surfactant Complexes in Drug Solubilisation/Stabilisation during Drug Release from Solid Dispersions. Pharmaceutical Research. 2012; 30(1): 290–302
Acknowledgements:The Madison & Lila Self Graduate Fellowship; George Wang, Ph. D, at Bristol Myers Squibb for running scanning electron microscopy (SEM).
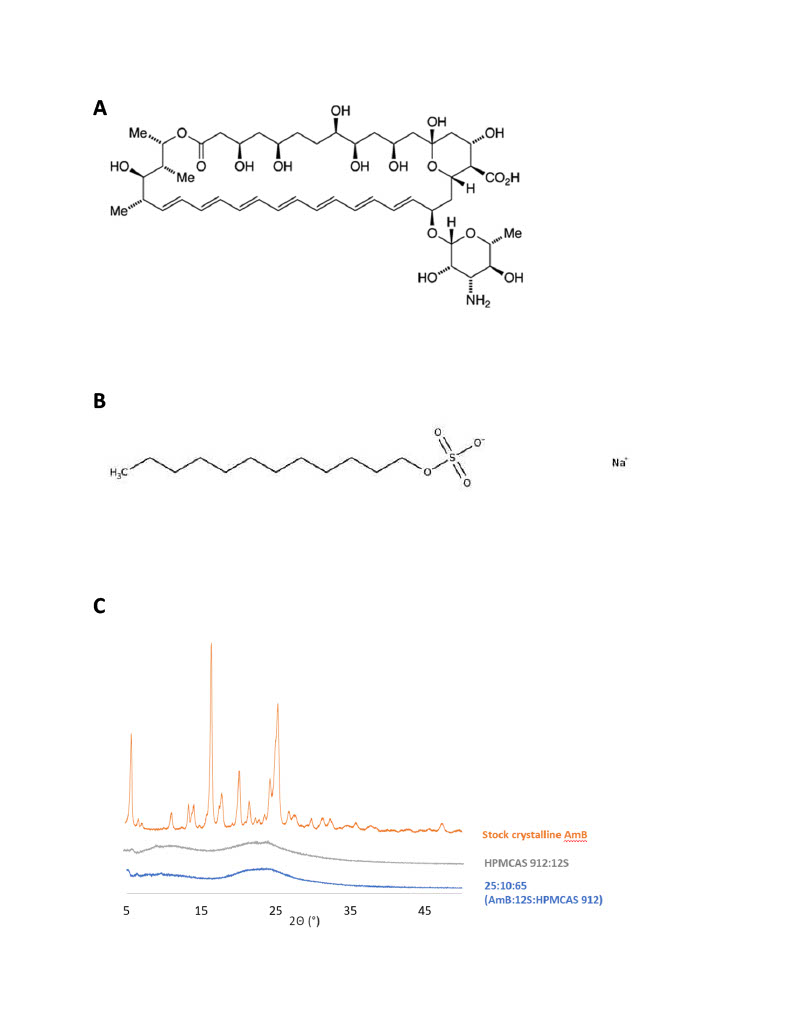 (A) Structure of AmB illustrating the long 14-carbon chain in close proximity with the cationic amine on the mycosamine sugar moiety. (B) Structure of sodium dodecyl sulfate (12S) for reference on the potential interactions possible between AmB and the anionic surfactants chosen. (C) PXRD data confirms the crystallinity of the starting AmB material, along with confirming the amorphous nature of the spray-dried products. The ternary formulation containing 25:10:65 (AmB:12S:HPMCAS 912) was used as a model formulation here.
(A) Structure of AmB illustrating the long 14-carbon chain in close proximity with the cationic amine on the mycosamine sugar moiety. (B) Structure of sodium dodecyl sulfate (12S) for reference on the potential interactions possible between AmB and the anionic surfactants chosen. (C) PXRD data confirms the crystallinity of the starting AmB material, along with confirming the amorphous nature of the spray-dried products. The ternary formulation containing 25:10:65 (AmB:12S:HPMCAS 912) was used as a model formulation here.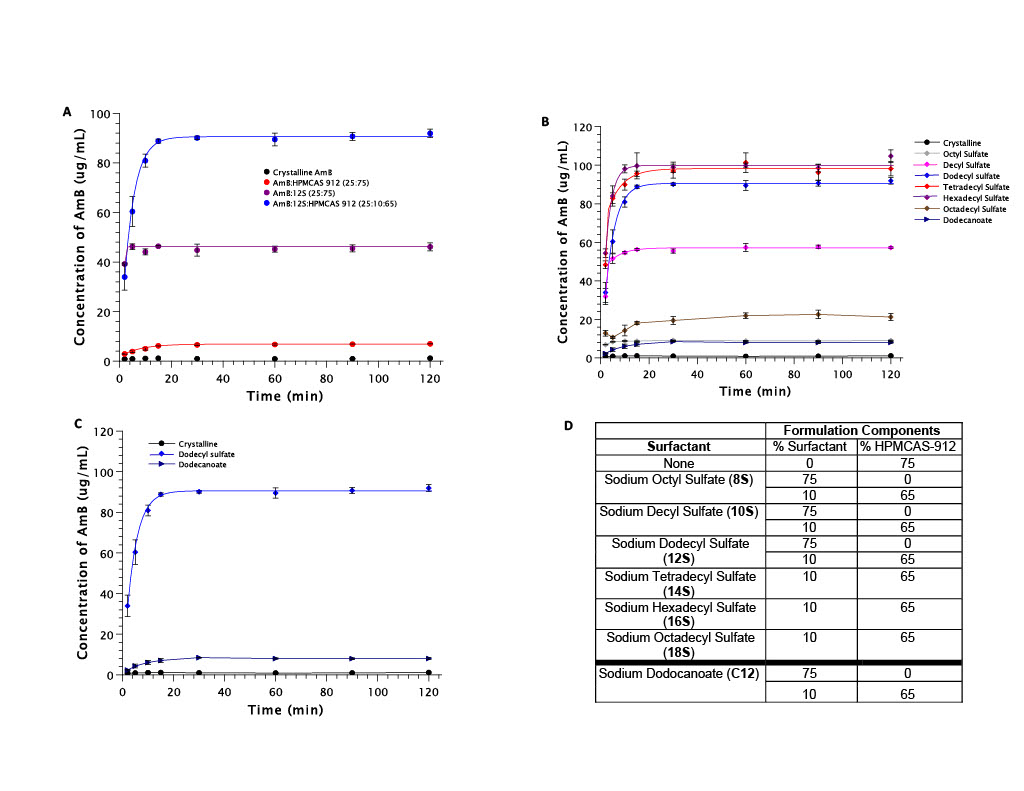 (A) Dissolution of AmB from ternary formulation containing both surfactant and polymer compared to dissolution of AmB from both binary formulations containing either surfactant or polymer. Dodecyl sulfate (12S) was used as the model surfactant. (B) Dissolution of AmB from all TERNARY formulations containing surfactants with varying carbon chain length. (C) Dissolution of AmB from ternary formulations containing two different surfactants with an equal carbon chain length (12 carbons) but a different functional group (sulfate group (dodecyl sulfate) vs carboxylate group (dodecanoate)). (D) Table of all spray-dried AmB ASD formulations with accompanying component percentage (w/w/w).
(A) Dissolution of AmB from ternary formulation containing both surfactant and polymer compared to dissolution of AmB from both binary formulations containing either surfactant or polymer. Dodecyl sulfate (12S) was used as the model surfactant. (B) Dissolution of AmB from all TERNARY formulations containing surfactants with varying carbon chain length. (C) Dissolution of AmB from ternary formulations containing two different surfactants with an equal carbon chain length (12 carbons) but a different functional group (sulfate group (dodecyl sulfate) vs carboxylate group (dodecanoate)). (D) Table of all spray-dried AmB ASD formulations with accompanying component percentage (w/w/w).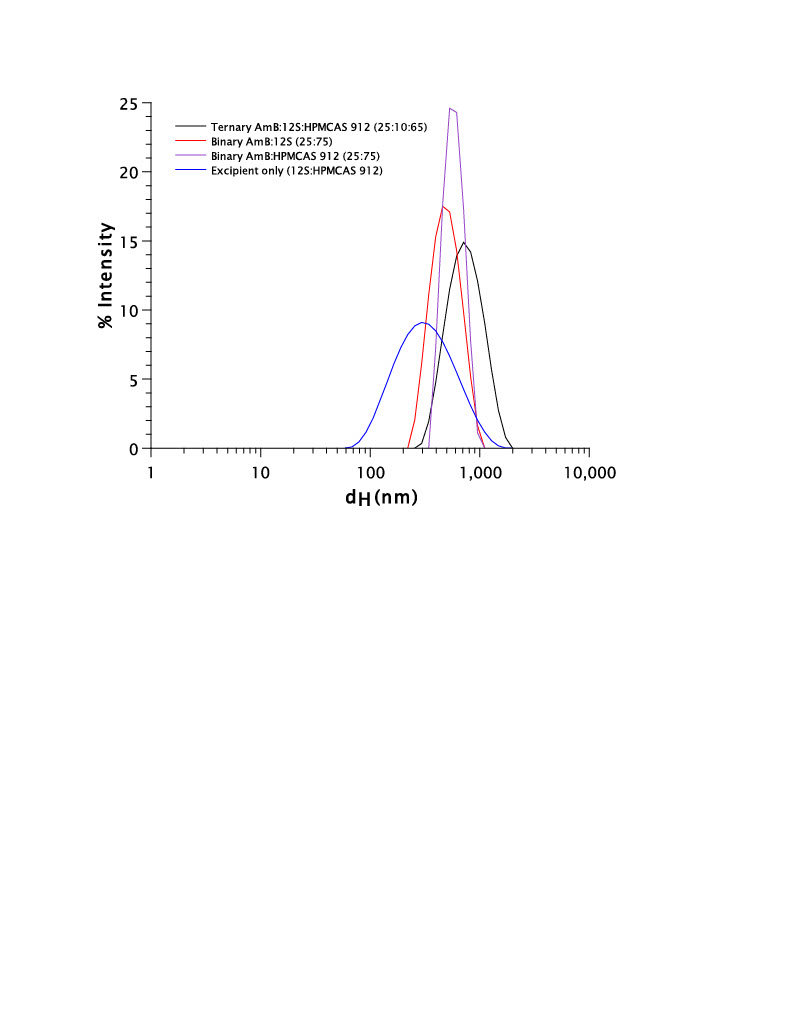 DLS profile of ternary formulation containing both surfactant and polymer compared to both binary formulations containing either surfactant or polymer. All three formulations were compared to the DLS profile of spray-dried excipients only (absence of AmB). Excipients are present at the same concentrations as they would be for the ternary formulation in solution.
DLS profile of ternary formulation containing both surfactant and polymer compared to both binary formulations containing either surfactant or polymer. All three formulations were compared to the DLS profile of spray-dried excipients only (absence of AmB). Excipients are present at the same concentrations as they would be for the ternary formulation in solution.