Formulation and Delivery - Biomolecular
Category: Poster Abstract
(W1030-02-08) Characterization of In Situ Forming Implants Using CT Imaging

Xinhao Lin, BS
Doctoral Candidate Research Assistant
University of Connecticut
Storrs, Connecticut, United States
Xinhao Lin, BS
Doctoral Candidate Research Assistant
University of Connecticut
Storrs, Connecticut, United States- NZ
Nour Al Zouabi
University of Connecticut
Storrs, Connecticut, United States - ZZ
Zixuan Zhen, MS
University of Connecticut
Storrs, Connecticut, United States - LW
Lauren E. Ward
University of North Carolina Chapel Hill
Chapel Hill, North Carolina, United States - HY
Hong Yuan, Ph.D.
University of North Carolina Chapel Hill
Chapel Hill, North Carolina, United States - MJ
Michael Jay, Ph.D.
University of North Carolina Chapel Hill
Chapel Hill, North Carolina, United States - AB
André O'Reilly Beringhs, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - BQ
Bin Qin, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - XL
Xiuling Lu, Ph.D.
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: In situ forming implants have attracted increasing attention for delivering proteins, peptides, and new therapeutic entities. In order to obtain improved understanding of implant formation and its impact on drug release, we developed a non-invasive imaging approach utilizing computed tomography (CT) to monitor the implant formation and morphology changes in vitro and in vivo.
Methods: ELIGARD formulation was used in this study. An X-ray CT contrast agent, iohexol, was included for facilitating the observation of implant formation using CT. To prepare the injectable formulation, poly(lactic-co-glycolic acid) (PLGA) copolymer (50:50, acid endcap, 25 kDa) was dissolved in N-methyl-2-pyrrolidone (NMP) and then iohexol and/or leuprolide acetate (LA) were added to the PLGA solution. For in vitro formed implants, 250 µL of the formulation were injected into vials with phosphate buffered saline (PBS; pH 7.4) and maintained in bath shaker at 37°C with the shaking speed of 80 rpm (n=3). The weight and volume of the implants were measured at each time point. In vitro release and scanning electron microscope (SEM) studies were also conducted under the same conditions to characterize release profiles and microstructures, respectively. For in vivo formed implants, the same volume of the formulation was administered subcutaneously to rats (n=5). CT images were obtained using the IVIS Spectrum CT system (PerkinElmer, USA) at specific time points after injection.
Results: Formulations with iohexol only showed high extent of solvent (NMP) burst release in the first 48 hours (66.94 ± 4.42 % and 75.83 ± 7.35 % for iohexol and NMP, respectively) (Fig. 1). This fast solvent exchange leads to the weight and volume decrease during the burst release. Addition of leuprolide acetate reduced the burst release of iohexol and NMP to be below 20%, and extended the iohexol release duration. Based on the CT images of in vitro formed implants, iohexol was condensed in the core for the formulation with iohexol only (Fig. 2). With addition of leuprolide acetate, the retardation of burst release resulted in a more evenly distributed iohexol with a thin layer of shell containing lower concentration of iohexol. Moreover, the addition of leuprolide acetate promoted the weight and volume increase of the implants, indicating an increased water influx after the initial burst release phase. Polymer degradation was observed as the porosity observed from SEM images. The timing of the polymer degradation was consistant with the second fast release period. The release profiles were well-aligned with the implant structures as reflected from CT and SEM images. For in vivo formed implants, similar morphology and inner structure of the implants were observed compared to the in vitro samples. The formulation with iohexol showed similar core-shell structure of the iohexol deposition as the in vitro formed implants at the first 2 days. However, the process of the implant formation and change of the inner structure in vivo were faster than in vitro.
Conclusion: Using iohexol for CT contrast assisted in determining the morphological characteristics of in situ-forming implants in real time during the implant formation process. Addition of leuprolide acetate changed the release profiles and deposition of iohexol both in vitro and in vivo. Properties of the inner structure and morphologies of the implants are correlated with the change of weight and polymer degradation. Comparing in vitro and in vivoformed implants, both show similar inner structure and morphology, but the in vivo environment accelerates the formation process and limits the size expansion of the implants.
Acknowledgements: The authors would like to acknowledge the U.S. Food and Drug Administration for financial support of this research (contract number:75F40120C00136).
Disclaimer: The views expressed in this abstract do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
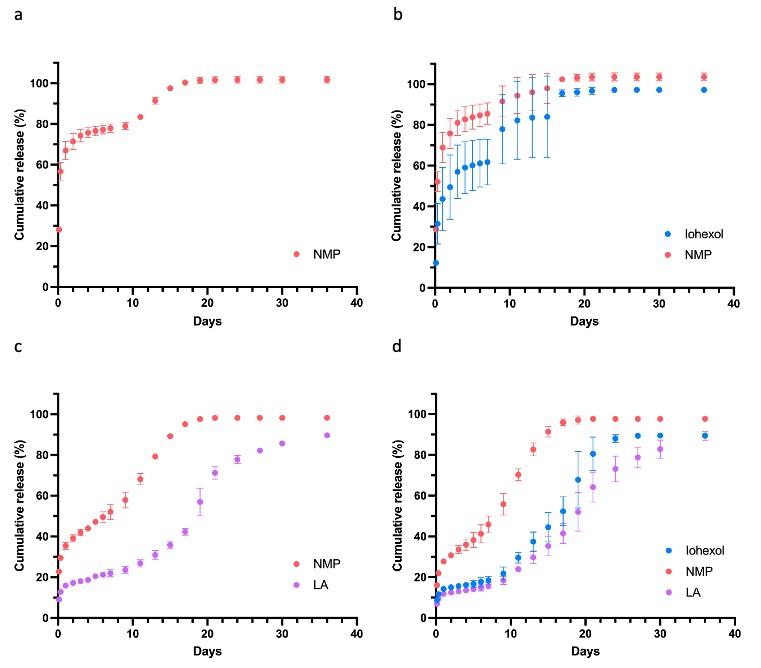 Fig. 1 In vitro release profiles of (a) placebo formulation, (b) formulation with only iohexol, (c) formulation with only leuprolide acetate and (d) formulation with both iohexol and leuprolide acetate. In vitro drug release tests were conducted under sink conditions in PBS at pH 7.4 and 37 °C (mean±SD, n=3).
Fig. 1 In vitro release profiles of (a) placebo formulation, (b) formulation with only iohexol, (c) formulation with only leuprolide acetate and (d) formulation with both iohexol and leuprolide acetate. In vitro drug release tests were conducted under sink conditions in PBS at pH 7.4 and 37 °C (mean±SD, n=3).  Fig. 2 CT images of in vitro formed implants. Representative transaxial section of the 3D CT images were collected for each implant at each time point.
Fig. 2 CT images of in vitro formed implants. Representative transaxial section of the 3D CT images were collected for each implant at each time point. 