Formulation and Delivery - Chemical
Category: Poster Abstract
(M0930-02-10) Dissolving Microarray Patches Loaded with Atorvastatin Microparticles for Intradermal Long-Acting Delivery
.jpg)
Yara A. Naser, Ph.D. (she/her/hers)
Postdoctoral research fellow
Queen's University Belfast
BELFAST, Northern Ireland, United Kingdom.jpg)
Yara A. Naser, Ph.D. (she/her/hers)
Postdoctoral research fellow
Queen's University Belfast
BELFAST, Northern Ireland, United Kingdom- LV
Lalitkumar K. Vora, Ph.D. (he/him/his)
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom - IT
Ismaiel Tekko, Ph.D. (he/him/his)
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom - kP
ke Peng, Ph.D. (she/her/hers)
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom - HM
Helen McCarthy, Ph.D. (they/them/theirs)
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom - RD
Ryan F. Donnelly, Ph.D. (he/him/his)
Queen's University Belfast
Belfast, Northern Ireland, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Due to their capacity to offer a continuous release of drugs after administration, prolonged-release dosage forms are growing in popularity amongst scientists. This enhances patient compliance and, as a result, their overall quality of life [1]. However, injections or implants are typically needed to achieve prolonged release lasting longer than 24 hours. These methods are intrusive and inappropriate for those suffering from needle phobia [2]. In this study, dissolving microarray patches (D-MAPs) containing atorvastatin (ATR) microparticles were created to deposit a therapeutic dose intradermally as a micro-depot for the future long-acting release of ATR over a period of two weeks.
Methods: Figure 1 illustrates the bilayer-casting method used to produce D-MAPs [3]. Hydrogel blends of poly(vinyl alcohol) (PVA) 9–10 kDa and poly(vinyl pyrrolidone) (PVP) 58 kDa, and ATR, constituted the first layer of the D-MAPs. Using a SpeedMixerTM at 3000 rpm for 5 minutes, the hydrogel blends were mixed. The second layer comprised PVP 360 kDa, glycerol, and water. These D-MAPs were evaluated in terms of their mechanical characteristics, drug content, and in situ insertion efficiency. Franz cells were used in a 24-hour ex vivo skin deposition study to determine the amount of ATR deposited intradermally. The in vivo study was conducted over 2 weeks using Sprague Dawley rats. Each rat received 4 D-MAPs, each containing 5 mg of ATR. A control group was given an oral suspension of ATR at a dose of 10 mg/rat. Plasma samples were obtained and analysed using a Waters® Triple Quadruple Tandem Mass Spectrometer (TQ MS-MS).
Results: D-MAPs were strong enough to penetrate skin-simulant Parafilm M® and excised neonatal porcine skin models with thumb pressure, and they could withstand up to 32 N compression force with a 10% height reduction. D-MAPs had an average drug loading of 5.15 ± 0.4 mg of ATR/MAP. Their tips dissolved in situ within 60 minutes and were completely inserted into the porcine skin. Skin deposition studies revealed that 1.3 ± 0.1 mg ATR was deposited in the skin 24 hours after the application of D-MAPs. In addition, 0.8 ± 0.4 mg ATR was measured in the receiver compartment of Franz-cells. As a result, the total amount of ATR delivered after 24 hours was found to be 2.0 ± 0.33 mg, representing 38.76.7% of the initial amount loaded per D-MAP. The 14-day in in vivo study revealed that ATR was detected and quantified throughout the whole study period. This shows that after applying D-MAPs, micro-depots form intradermally, allowing for sustained ATR release throughout the research period. This is the first study to prove the long-acting transdermal delivery of ATR using D-MAPs over two weeks after a single dose. Figure 2 depicts the ATR plasma profile in both D-MAPs and control cohorts over 2 weeks.
Conclusion: These findings reveal D-MAPs' ability to deposit hydrophobic ATR intradermally for subsequent long-acting release. D-MAPs improved the transdermal delivery of the hydrophobic drug ATR. Ex-vivo skin deposition experiments revealed that microparticle-loaded D-MAPs could successfully deliver ATR across full-thickness porcine skin. For the first time, the in vivo study demonstrated the efficacy of D-MAPs in the long-acting administration of ATR over two weeks. This innovative technology provides a potential, less invasive, long-acting alternative ATR delivery system capable of improving patient compliance and therapeutic outcomes. A future pharmacodynamic investigation could be conducted, to evaluate the correlation between drug availability and treatment efficacy in rats and other suitable animal models afterwards prior to upscaling to a clinical level.
References: Reference:
1. Vijayakumar, V. and K.G. Subramanian, Drug carriers, polymers as: Synthesis, characterization, and in vitro evaluation, in Encyclopedia of Biomedical Polymers and Polymeric Biomaterials. 2016. p. 1-28.
2. Nikita P. Karode, V.D.P., Himanshu K. Solanki, Girish K. Jani, Sustained release injectable formulations: Its rationale, recent progress and advancement. World Journal of Pharmacy and Pharmaceutical Sciences, 2015. 4(04): p. 702 - 722.
3. Vora, L.K., et al., Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. Journal of Controlled Release, 2017. 265: p. 93-101.
.jpg) Figure 1. A schematic representation illustrating the steps required in the fabrication of high-density moulds and D-MAPs.
Figure 1. A schematic representation illustrating the steps required in the fabrication of high-density moulds and D-MAPs.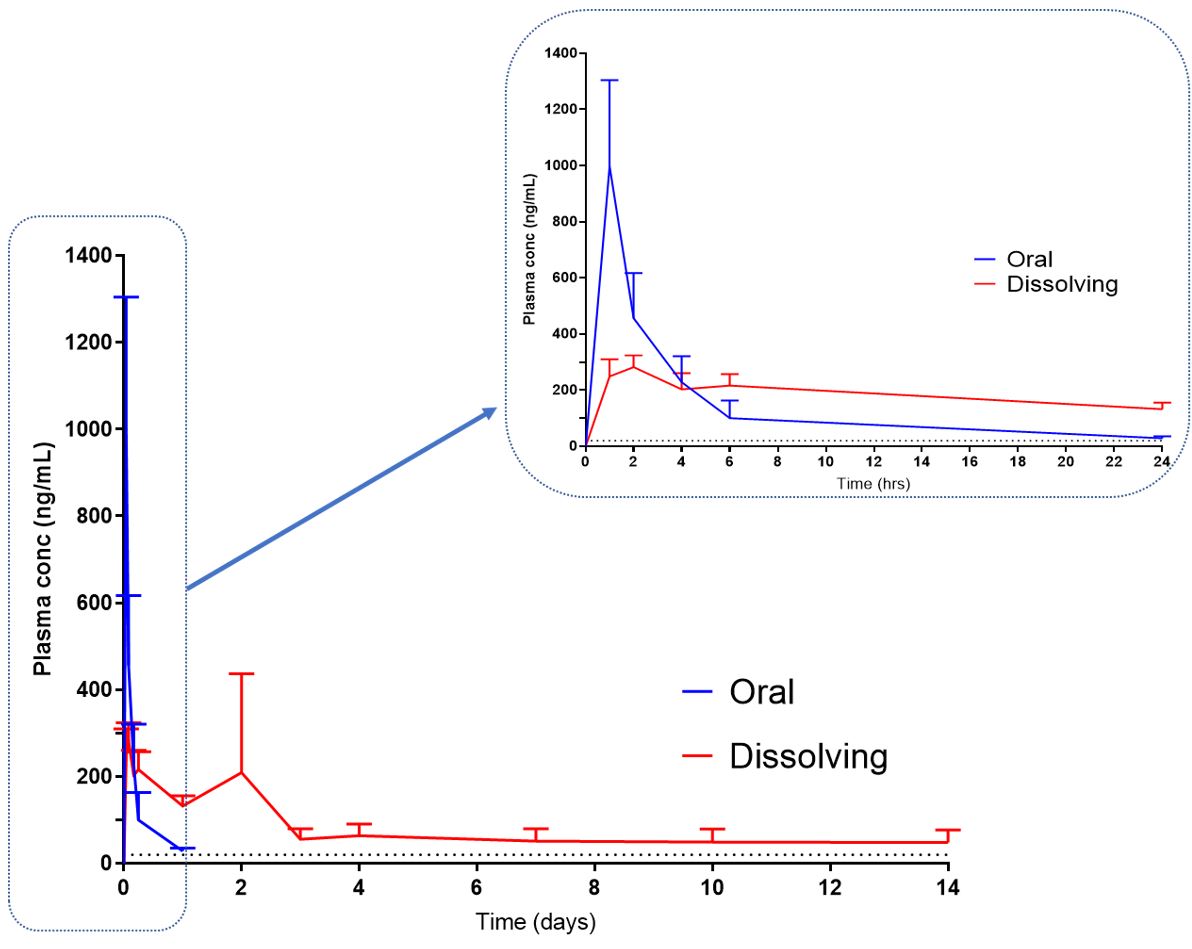 Figure 2. ATR plasma profiles of the rats from the oral and D-MAPs cohort following the in vivo study for 2 weeks. (Means + SD, n=6 at 1, 2, 4, and 6 h, n=12 at the remaining time points) The black dashed line represents the therapeutically relevant ATR plasma concentration in humans (20 ng/mL)
Figure 2. ATR plasma profiles of the rats from the oral and D-MAPs cohort following the in vivo study for 2 weeks. (Means + SD, n=6 at 1, 2, 4, and 6 h, n=12 at the remaining time points) The black dashed line represents the therapeutically relevant ATR plasma concentration in humans (20 ng/mL)