Bioanalytics - Biomolecular
Category: Late Breaking Poster Abstract
(T1530-06-38) Out of Endotoxin Box: Rethinking Pyrogens
Tuesday, October 24, 2023
3:30 PM - 4:30 PM ET
- DM
Djikolngar Maouyo, Ph.D. (he/him/his)
PyroDex LLC
Baltimore, Maryland, United States - DM
Djikolngar Maouyo, Ph.D. (he/him/his)
PyroDex LLC
Baltimore, Maryland, United States
Presenting Author(s)
Main Author(s)
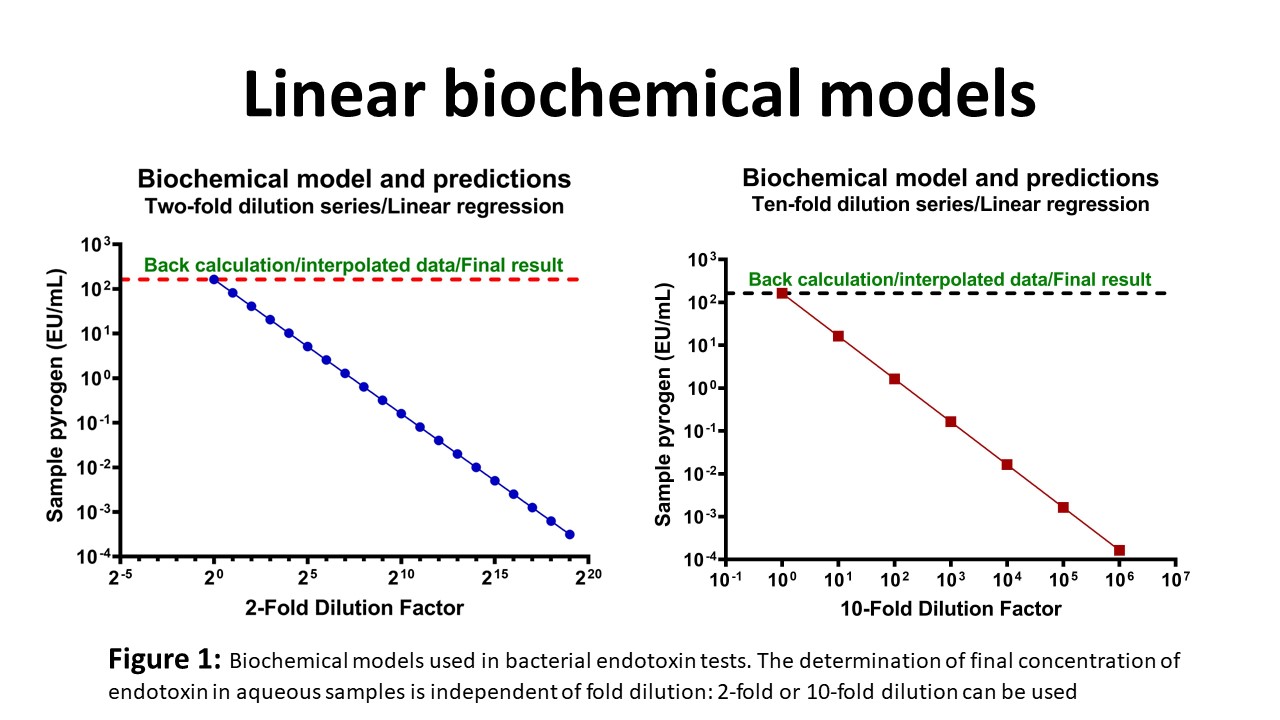
Purpose: The MAT requires two-fold serial dilutions of samples to establish the secretory pattern of the pro-inflammatory cytokine biomarker. The main goal of this study is to show new concepts and principles for the determination of pyrogen contents with ineluctable accuracy, for safer medical parenteral drugs and implantable devices, for better treatment and more live saved.
Methods: The monocyte activation test (MAT) used in this study is based on cryopreserved peripheral blood mononuclear cells (PBMCs). PBMC are exposed to serial concentrations of the reference standard endotoxin purchased from the US Pharmacopeia store, and the lipoteichoic acid (LTA) from Invivogen. Interleukin-6 (IL-6) from the cell supernatant collected after 20-hour activation at 37°C. IL-6 concentrations from supernatant samples are quantified using a microfluidic system-based ELISA, Simple Plex and Ella, from ProteinSimple/Biotechne.
Results: The endotoxin-induced secretory pattern of interleukin-6 (IL-6) used for pyrogen detection is consistently sigmoidal, independently of cell density, defining a characteristic biological response type designated “endotoxin-type response.” The LTA-induced secretory pattern (“LTA-type monocytic response”) is characteristically biphasic (maximal response between the high and low dilutions). The pattern showed one unique bifurcation/inflection point at optimal dilution. Other dilutions corresponding to symmetric or asymmetric paired concentrations are considered equivocal and invalid, and cannot be used for quantification of pyrogen contents. The inflection point corresponds to the unique valid dilution/optimal concentration.
Conclusion: Biological responses to endotoxins and non-endotoxin pyrogens are non-linear, suggesting that the establishment of secretory pattern of cytokines used in the monocyte activation test is required for an ineluctable accuracy in safety testing of parenteral drugs and implantable medical devices.
Acknowledgements: The author is founder of PyroDex LLC, a company fully dedicated to offer safety testing services based specifically on the Monocyte Activation Test that does not rely on animal products.
.jpg)
.jpg)
