Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(T1530-12-78) Preclinical Evaluation of Brinzolamide Ophthalmic Suspensions with Variations in Critical Quality Attributes and Considerations for Pharmacokinetic/Pharmacodynamic Study Designs

Andre O'Reilly Beringhs, PhD (he/him/his)
Pharmacologist
US Food and Drug Administration
Germantown, Maryland, United States
Andre O'Reilly Beringhs, PhD (he/him/his)
Pharmacologist
US Food and Drug Administration
Germantown, Maryland, United States- VN
Vatsala Naageshwaran, B.S.
Pharmaron (Exton) Lab Services LLC
San Diego, California, United States - GG
Glenwood Gum, Ph.D.
Pharmaron (Exton) Lab Services LLC
San Diego, California, United States - SM
Spundana Malla, Ph.D.
Pharmaron (Exton) Lab Services LLC
San Diego, California, United States - AV
Anh Vo, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - WS
William Smith, Ph.D.
US Food and Drug Administration
Silver Springs, Maryland, United States 
Ming-Liang Tan, PhD (he/him/his)
Sr. Pharmacokineticist
US Food and Drug Administration
Silver Spring, Maryland, United States- AB
Andrew Babiskin, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - XX
Xiaoming Xu, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Darby Kozak, Ph.D.
Deputy Division Director
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Brinzolamide ophthalmic suspensions are indicated for treatment of elevated intraocular pressure (IOP), a major risk factor for optic nerve damage and glaucomatous visual field loss. In an effort to understand the influence of critical quality attributes (CQAs) of these ophthalmic suspensions on their pharmacokinetic/pharmacodynamic (PK/PD) performance, the impact of variations in select CQAs (i.e., viscosity and particle size distribution, PSD) on the PK/PD of brinzolamide was investigated in a rabbit model.
Methods: Brinzolamide ophthalmic suspensions 1% were prepared following the same qualitative and quantitative composition (± 5%) as the Reference Listed Drug (RLD) product AZOPT (NDA 020816). Variations in PSD and viscosity were achieved by shear using a planetary centrifugal miller, and by tailoring the concentration of thickening agent, respectively. PK/PD studies were parallel with single-dose or multi-dose (daily) topical ophthalmic instillations of brinzolamide ophthalmic suspensions (0.5 mg/50 µL per eye) in both eyes of New Zealand White (NZW) rabbits. Animals were euthanized at select time-points post-dosing, and relevant ocular tissues were harvested for drug quantification via LC-MS/MS. PK parameters were determined using a non-compartmental analysis (NCA) model (Phoenix WinNonlin 8.0). IOP measurements were conducted via applanation tonometer (Reichter Model 30) at select time-points post-dosing.
Results: Test formulations (BRZ_001 – 005) and RLD AZOPT were characterized for PSD and viscosity over a range of shear rates (Table 1). In addition, the ranges of pH and osmolality of all formulations were 7.5 – 7.9 and 258 – 289 mOsm/kg, respectively. Based on the mean concentration-time profiles in relevant ocular compartments, i.e., aqueous humor (AH) and iris-ciliary body (ICB), no clear correlation between the CQAs and the ocular pharmacokinetics of brinzolamide was observed at the first day of dosing (single-dose). Similar observations were noted for the pharmacodynamic assessment. In addition, multi-dosing for 14 days also did not yield discirnable results. Inter- and intra-subject variability analyses of the first-day PK data indicate the intra-animal variability of Cmax in AH was substantially lower than the total variability. Thus, although no clear correlation between CQAs was established in the current studies, the variability of the studies can inform the design of more appropriately powered PK studies.
Conclusion: The high variability observed in PK profiles creates challenges evaluating the impact of CQAs of brinzolamide ophthalmic suspensions on PK. There was no clear correlation between changes in CQAs and PK/PD of brinzolamide suspensions following single- and multi-dose parallel study designs. The variability of the data indicates alternative study designs may be considered. For instance, the lower intra-animal variability may suggest a paired-eye study design may be superior to the parallel design used in this study for the purpose of determining drug product performance equivalence.
Acknowledgements: This study was conducted at Pharmaron US Lab Services with funding from the U.S. Food and Drug Administration (IDIQ Contract 75F40119D10024-75F40120F19002). The views expressed in this abstract do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
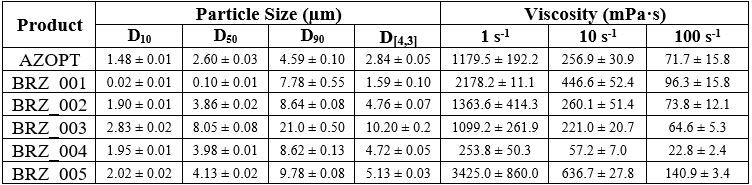 Table 1. Physicochemical properties of brinzolamide ophthalmic suspensions (mean ± standard deviation; n=3).
Table 1. Physicochemical properties of brinzolamide ophthalmic suspensions (mean ± standard deviation; n=3).