Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(T1430-09-59) Regulatory Experience with Continuous Manufacturing and Real Time Release Testing for Dissolution in New Drug Applications
Tuesday, October 24, 2023
2:30 PM - 3:30 PM ET

Maziar Kakhi, PhD (he/him/his)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States
Maziar Kakhi, PhD (he/him/his)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States- JL
Jing Li, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - AD
Angelica Dorantes, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Regulatory submissions to the FDA involving the use of continuous manufacturing (CM) and/or real-time release testing in conjunction with a model for predicting in vitro dissolution testing (RTRT-D) were identified. The submissions were examined from a biopharmaceutics perspective to highlight commonly occurring issues which the FDA’s assessment teams identified with the proposed use of CM and/or RTRT-D. The objective of this abstract is to provide recommendations for best practices that will help advance the field by (i) generating greater opportunities for (drug) Applicants to benefit from the implementation of advanced manufacturing approaches, (ii) improving high quality regulatory submissions involving CM and RTRT-D, and thus (iii) lessening the regulatory review burden.
Methods: The sample of submissions in this abstract is the result of exploratory searches using FDA-internal databases. All of the submissions discussed in this study represent drug products (DPs) which are orally administered immediate release tablets. Furthermore, the applicants applied Quality by design (QbD) principles, namely (i) a quality risk assessment for identifying the critical material attributes (CMAs) and critical process parameters (CPPs) which might impact the critical quality attributes (CQAs), (ii) design of experiments (DoE) to support a quantitative mapping of the relationship between the CMAs/CPPs to the CQAs, and (iii) process analytical technology and chemometric modeling coupled to a control strategy.
Results: Table 1 provides an overview of the major deficiencies identified for each of the case studies. Figure 1 expresses the frequency of occurrence of major deficiencies aggregated over all case studies. Table 1 shows that of the 8 case studies involving RTRT-D submissions, only three were approved, indicating a low approval percentage. Case studies 1 and 3 demonstrated that the development and validation of an RTRT-D needs a well understood, canonically sound and discriminating dissolution method. For case study 1, the dissolution method exhibited very large variability whose source was unclear impacting the credibility of the proposed DoE data for model calibration and validation purposes. For case study 3, the proposed dissolution method’s atypical and overdiscriminating behavior was deemed inadequate for developing an RTRT-D. In this instance, the dissolution method exhibited an increase of dissolution rate with increasing particle size, tablet compression, and uncoated tablet density. Also, the dissolution was overdiscriminating for the presence of non-functional coating versus uncoated tablets. In case study 2 particle size control of the drug substance was proposed as a surrogate for dissolution testing, which failed to provide a measure for dissolution, in both the rate and extent of release. Case study 4 specified an RTRT-D for predicting Q30, which still fell short of a complete dissolution profile. The dissolution model contained hardness as a predictor variable which is itself dependent on CPPs and CMAs, thereby confounding the sought-after link between cause (variation of CPPs and CMAs) and effect (dissolution). Certain CMAs and CPPs were proposed to be tightly controlled through in-process controls instead of being included in DoE as input parameters. Such an approach limits the robustness of the RTRT-D to a narrower region of the design space and in its ability to identify out-of-specification DP. To address this, the applicant agreed to perform in vitro dissolution testing for deviation during the course of manufacture outside of the tightly controlled specifications for the CMAs/CPPs excluded from the RTRT-D. The in vitro dissolution acceptance criterion was proposed as the RTRT-D’s model validation acceptance criterion (MVAC). RTRT-Ds based on batch-averaged mean values of predictor variables were all found deficient in accounting for intra-batch variability. This is where tablet selection at periodic time intervals throughout a manufacturing run using a stratified sampling plan becomes critical. The design of such a plan (e.g., with respect to location, frequency, sample size, and statistical criteria for acceptance) would need to take into consideration the process dynamics, total run time, batch size and potential for transient disturbances or process variability. Case study 9 involved no major deficiencies. The RTRT-D was found to be acceptable largely because, 1)justification was provided for the selection of input parameters for the RTRT-D; 2) Calibration and verification of the RTRT-D was performed with a robust dissolution data set with an a priori defined MVAC; 3) For batch release, the use of segment averages (based on stratified sampling) assured that intra-batch variability could be accounted for; 4) Examples demonstrating the RTRT-D’s ability to detect non-conforming batches were presented.
Conclusion: In this study, several regulatory case studies were presented involving CM and RTRT-D with the aim of highlighting commonly occurring biopharmaceutics deficiencies.
Acknowledgements: This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
.jpg) Table 1: Summary of major deficiencies related to RTRT-D and sampling strategy in regulatory case studies. Key: w/d = Withdrawn; CR = Complete Response; A = Approved; CM = Continuous manufacturing; BM = Batch manufacturing. Column “BCS” refers to reported classification from Applicant, not FDA’s official BCS designation.
Table 1: Summary of major deficiencies related to RTRT-D and sampling strategy in regulatory case studies. Key: w/d = Withdrawn; CR = Complete Response; A = Approved; CM = Continuous manufacturing; BM = Batch manufacturing. Column “BCS” refers to reported classification from Applicant, not FDA’s official BCS designation.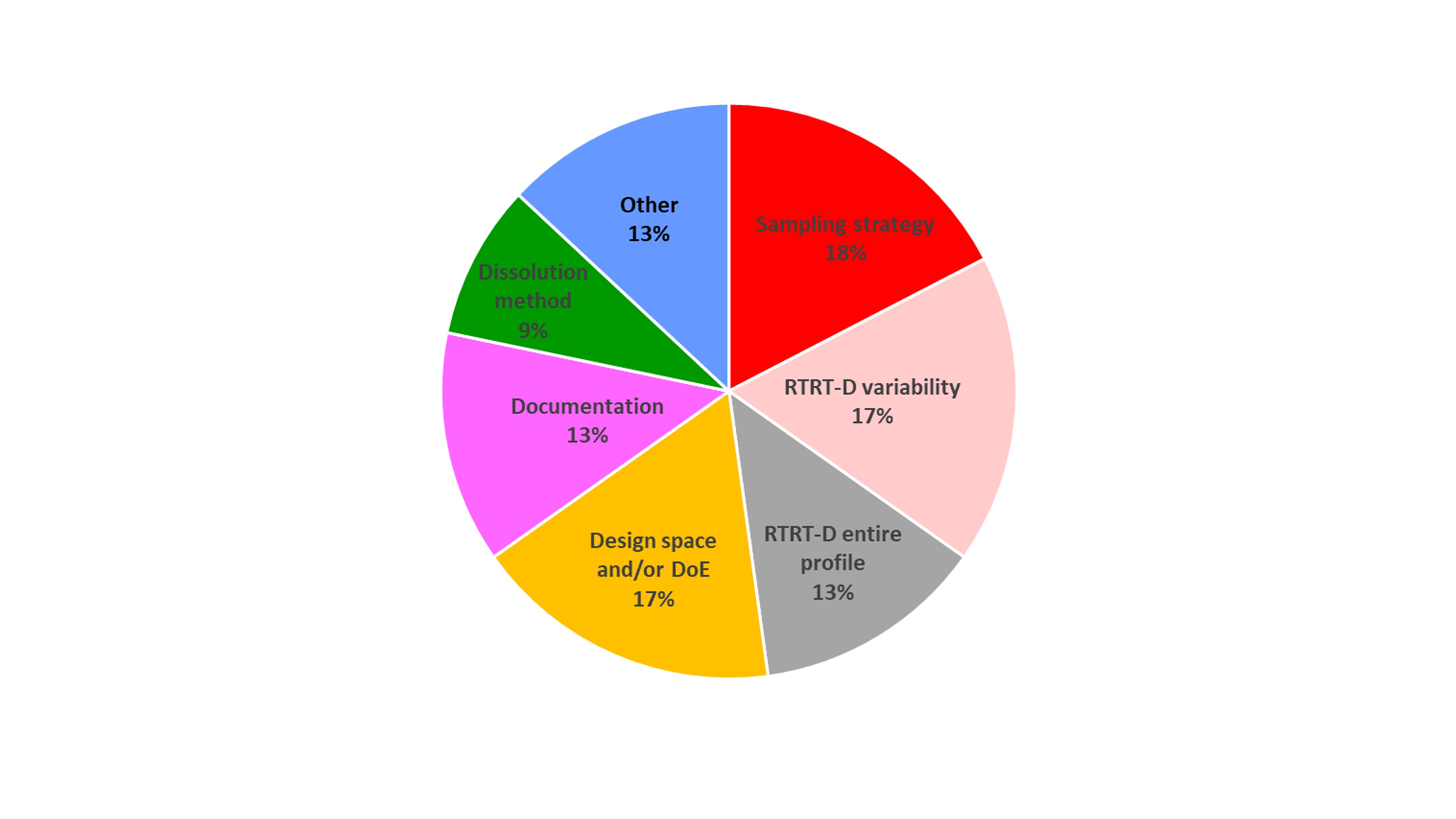 Figure 1: Frequency of occurrence major deficiencies aggregated over all case studies presented in Table 1
Figure 1: Frequency of occurrence major deficiencies aggregated over all case studies presented in Table 1