Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(T1030-09-62) Interconversion Kinetic Study to Assess Class II Atropisomeric Drug Development Challenges
Tuesday, October 24, 2023
10:30 AM - 11:30 AM ET
- YX
Yuling Xie, Ph.D. (she/her/hers)
Vertex Pharmaceuticals, Inc.
San Diego, California, United States - YX
Yuling Xie, Ph.D. (she/her/hers)
Vertex Pharmaceuticals, Inc.
San Diego, California, United States - OG
Ole Gron
Vertex Pharmaceuticals, Inc.
San Diego, California, United States - MS
Mark Strohmeier (he/him/his)
Vertex Pharmaceuticals, Inc.
Boston, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Atropisomers are stereoisomers resulting from hindered bond rotation that introduces time-dependent inversion of chirality. As atropisomerism is becoming prevalent in modern drug discovery, evaluation of the interconversion kinetics of atropoisomeric drug candidates is critical to provide risk assessment and mitigation strategies of potential development challenges of the drugs from both early drug discovery and later drug development.
Methods: In this work, we evaluated the interconversion kinetics of atropoisomeric a drug candidate by monitoring the peak area% difference of the two atropisomeric peaks using chiral HPLC with DAD. The change in relative peak area% was fitted to a first order reaction to calculate interconversion rates at a specific temperature. The rates were determined at a range of temperatures for the drug candidate. Through fitting of the rates at specific temperatures to Eyring-Polanyi equation, the interconversion activation parameters (free enthalpy ΔH‡ and entropy ΔS‡) can be extrapolated from the slope and intercept, allowing the determination of rotational energy barrier (free energy ΔG‡) at specific temperatures. We then calculated the energy barrier for this candidate in DMSO solution and as solid form ranging from amorphous drug substance (DS), neat crystalline DS to Spray Dried Dispersions (SDD).
Results: We determined that the rotational energy barrier ΔG‡ of our drug candidate in DMSO solution is 29.7 kcal mol-1 at 25 ˚C, which falls into Class II atropisomer (20-30 kcal mol-1). In addition, the energy barrier increases in order DMSO solution< SDD Solid< Neat Amorphous DS Solid< Neat Crystalline DS Solid. Using the calculated rotational energy barrier for SDD, 30.4 kcal mol-1 at 25 ˚C, it can be estimated that there will be ~1% conversion after 1 year storage at 25 ˚C, and >3% conversion after 3-month storage at 40 ˚C. In comparison, DS solids have higher rotational energy barrier at 25 ˚C which fall into Class III atropisomer ( >30 kcal mol-1) and can exist stable as a single atropisomer (e.g it would take >7 years to reach ~0.5% conversion for the neat amorphous DS solid).
Conclusion: Our drug candidate is a Class II atropisomer in DMSO solution and as SDD solid, which shows some stability concern due to interconversion in the order the months at 25 ˚C, lowering the storage temperature to 5 ˚C will greatly slow down the interconversion and maintain the chiral purity in years. The drug candidate as DS is much more stable and experience rate of interconversion in the order of years at 25 ˚C.
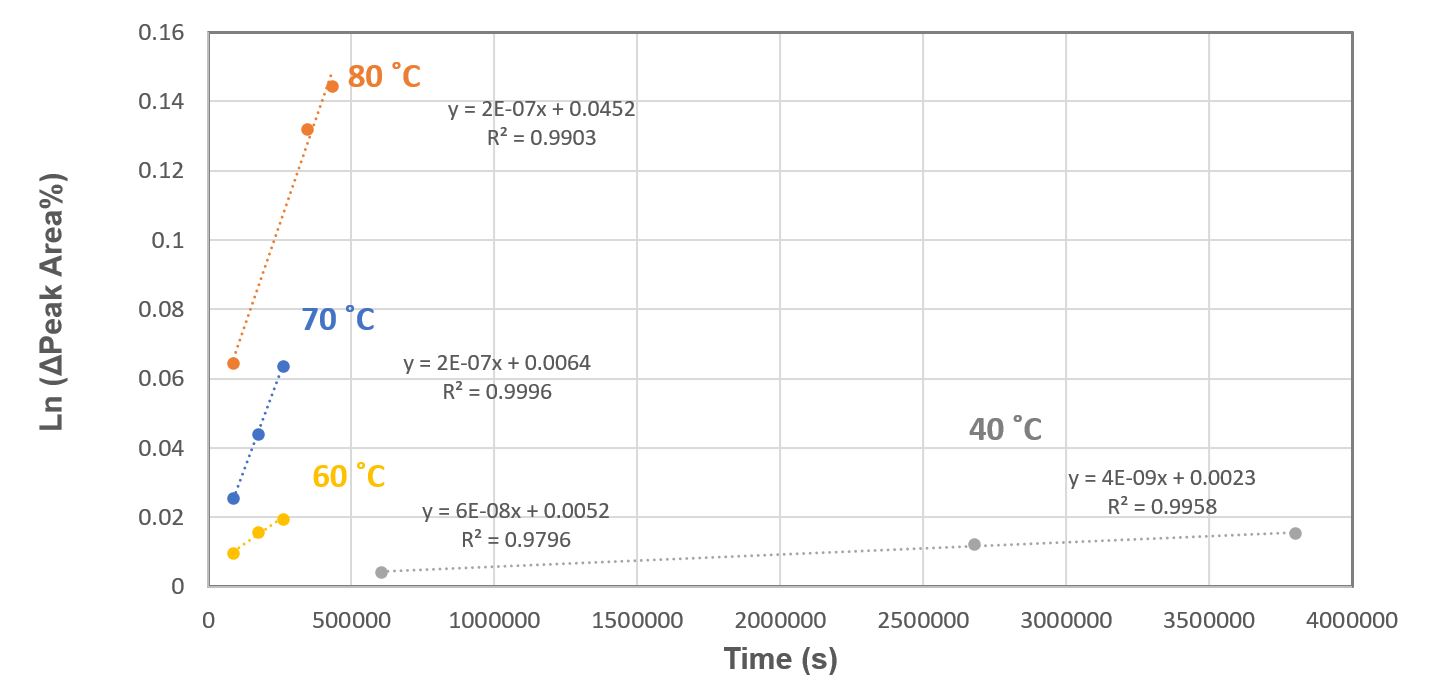 Figure 1: Drug SDD solid interconversion kinetic study: first order reaction fitting based on the HPLC-DAD peak area% difference and the two atropoisomeric peaks.
Figure 1: Drug SDD solid interconversion kinetic study: first order reaction fitting based on the HPLC-DAD peak area% difference and the two atropoisomeric peaks.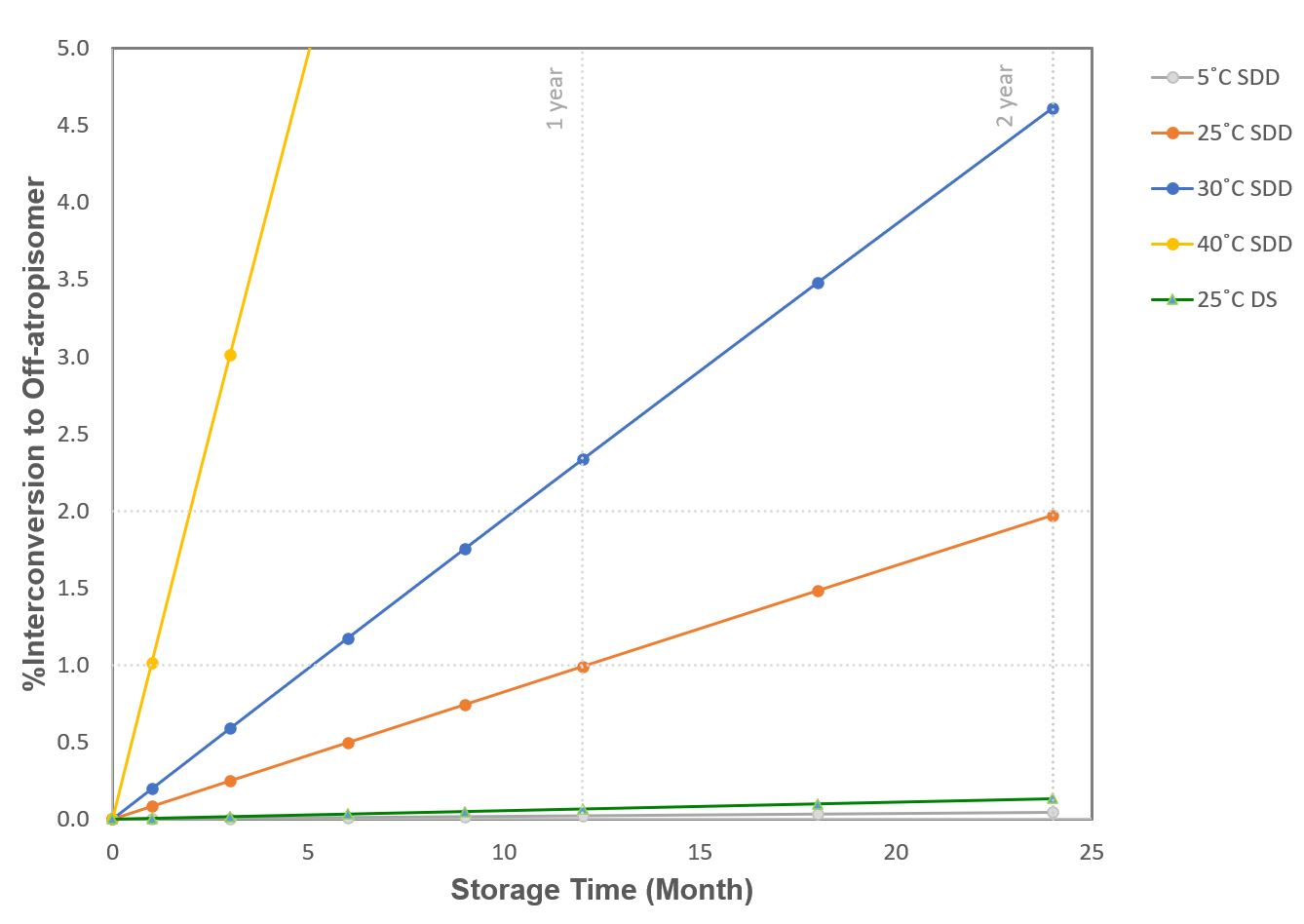 Figure 2: Interconversion to off-atropisomer in 24 Months for drug SDD solid at varying temperatures and DS solid at 25˚C for comparison.
Figure 2: Interconversion to off-atropisomer in 24 Months for drug SDD solid at varying temperatures and DS solid at 25˚C for comparison.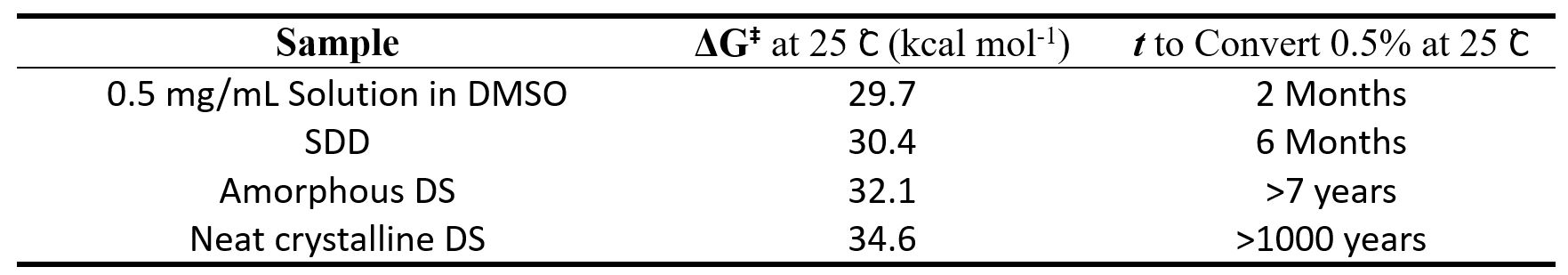 Table 1: Summary of rotational energy barrier (ΔG‡) and estimated time (t) to convert from single atropisomer (assuming 100% starting chiral purity) to form 0.5% the off-atropoisomer.
Table 1: Summary of rotational energy barrier (ΔG‡) and estimated time (t) to convert from single atropisomer (assuming 100% starting chiral purity) to form 0.5% the off-atropoisomer.