Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(T0930-03-19) Temperature-Dependent Terahertz Time-Domain Spectroscopy as a Tool to Predict Crystallisation Tendencies of Amorphous Drugs
Tuesday, October 24, 2023
9:30 AM - 10:30 AM ET
- HM
Haseeb Mahmood, M.S.
University of Cambridge
CAMBRIDGE, England, United Kingdom - HM
Haseeb Mahmood, M.S.
University of Cambridge
CAMBRIDGE, England, United Kingdom
Presenting Author(s)
Main Author(s)
Purpose: Amorphous drugs offer superior biopharmaceutical properties in comparison to their crystalline counterparts, particularly regarding dissolution rates and kinetic solubility. In the pharmaceutical industry, polymers are often incorporated to hinder crystallization and improve the stability of amorphous drugs. Several factors influence crystallisation tendency – including polymer loading, bond interactions and molecular flexibility – but predicting the crystallisation propensity remains a challenge. As such, terahertz time-domain spectroscopy (THz-TDS) is suggested as a characterisation technique to assess crystalline propensity of amorphous solid dispersions (ASDs) due to its ability to measure molecular mobility – in terms of glass transition temperatures – which has proven correlations to amorphous stability. ASDs of felodipine and indomethacin - incorporating hydroxypropylmethlycellulose acetate succinate (HPMCAS) as the polymer of choice - were prepared and analysed, with the purpose of the investigation to assess the capabilities of THz-TDS in providing information on drug stability; with particular focus on the impact of polymer loading.
Methods: Drug and polymer samples, with polymer loading varying from 10-70%, were first mixed in a cryomill to allow easier attainment of an amorphous state. The mixed samples were then melted in between quartz windows at high temperatures using MeltPrep Vacuum Compression Molding (VCM), a form of hot melt extrusion, before being cooled rapidly in an ice bath to yield dispersions in the form of thin, cylindrical solids of roughly 150 μm sample thickness. For characterisation, the samples were measured from 80 K to 420 K using THz-TDS - with two quartz windows as reference.
Results: As is demonstrated in Figure 1a for the case of felodipine, amorphous samples were successfully prepared. The room temperature measurements show no clear peaks at specific frequencies due to the absence of coherent long-range motions in the molecular matrix. Analysing at 1 THz yields the plot presented in Figure 1b. As temperature increases, the absorption also increases as a result of the rise in available thermal energy facilitating greater molecular mobility. In terms of the influence of polymer loading, a notable observation is the almost identical absorption trends for polymer loadings of 30% and 50% - demonstrated with more clarity in Figure 2a. This indicates a potential critical polymer loading point, beyond which further increase in polymer loading has minimal impact on absorption. Conducting measurements at more precise loadings between these values would yield a specific critical polymer loading that could then be compared between the different drugs for further insight on stability. At 70% polymer loading however, the high absorption is attributed to moisture in the sample due to inefficient purging of the sample area for that run: highlighting the sensitivity of THz-TDS to the presence of water. Analysing the transition regions, as shown by the lines drawn to guide the eye in Figure 2a, clearly highlight the primary glass transition temperature termed Tg,a. The values obtained are in line with literature values of the calorimetric glass transition temperature, demonstrating the capability of THz-TDS in identifying this. Observing the regions in the 10% polymer loading of felodipine in Figure 2b, we see that there is potential for THz-TDS data to also highlight glass transitions at lower temperatures in line with the local mobility (Tg,b). However, experimental repeats are required to combat fluctuations and obtain clearer data at these reduced temperatures and is the next step. Doing so would yield further information on drug stability and provide focus on transitions at lower temperatures not currently available in literature. For indomethacin, Figure 3 again emphasises similarities in absorption spectra for polymer loadings of 30% and 50%. Again, further experiments will be conducted to confirm the data and allow more conclusive remarks on observed trends and comparisons between the different systems and drugs.
Conclusion: The results thus far have demonstrated the capabilities of THz-TDS in identifying the calorimetric glass transition temperature in line with literature, with these values a reflection on the global molecular mobility. Early results indicate the potential to also identify the transition regions associated with local mobility, but further experiments are required to provide concise data at these lower temperatures. Moreover, for both felodipine and indomethacin, the measurements suggest a critical polymer loading value between 30% and 50% – with such information useful in optimising drug stability. As such, we see the potential of THz-TDS in providing valuable information on the crystallisation propensity of drug-polymer systems – with the next step to conduct more experiments and repeats and eventually draw comparisons between different drugs. Fundamentally, we would hope to better understand the influence of properties such as flexibility and hydrogen bonding.
Acknowledgements: I'd like to acknowledge my research group, the Terahertz Applications Group at the University of Cambridge, as well as my funders AstraZeneca.
.jpg) Figure 1. Absorption coefficient of felodipine with varying polymer loading, at a) room temperature and b) 1 THz with increasing temperature.
Figure 1. Absorption coefficient of felodipine with varying polymer loading, at a) room temperature and b) 1 THz with increasing temperature.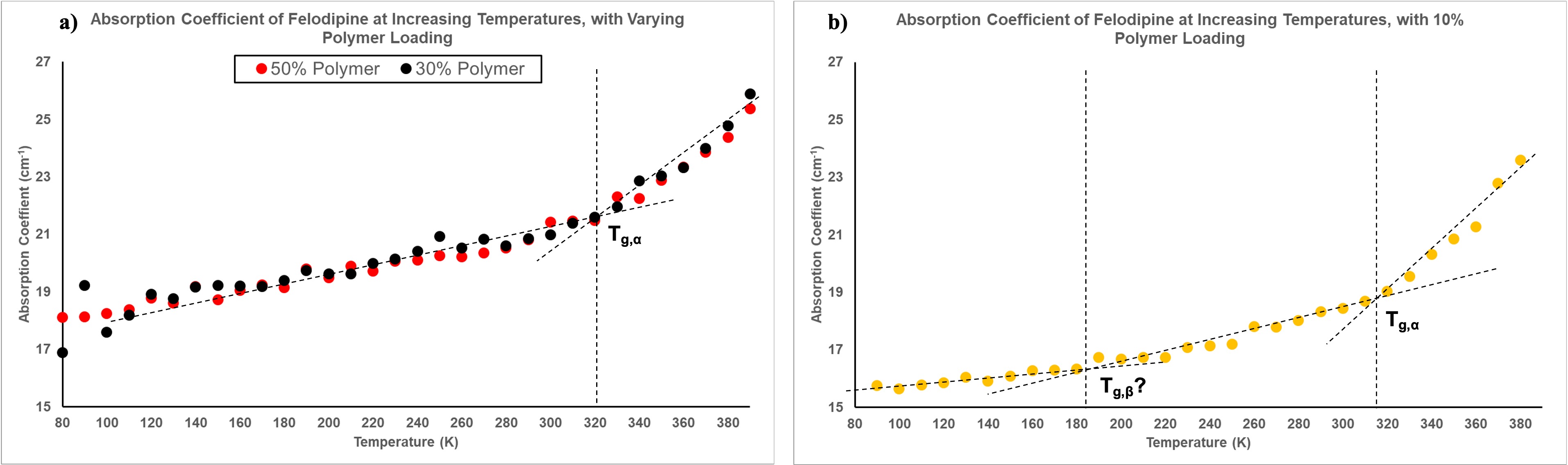 Figure 2. Transition regions of felodipine with a) 30% and 50% polymer loading and b) 10% polymer loading, analysed at 1 THz.
Figure 2. Transition regions of felodipine with a) 30% and 50% polymer loading and b) 10% polymer loading, analysed at 1 THz.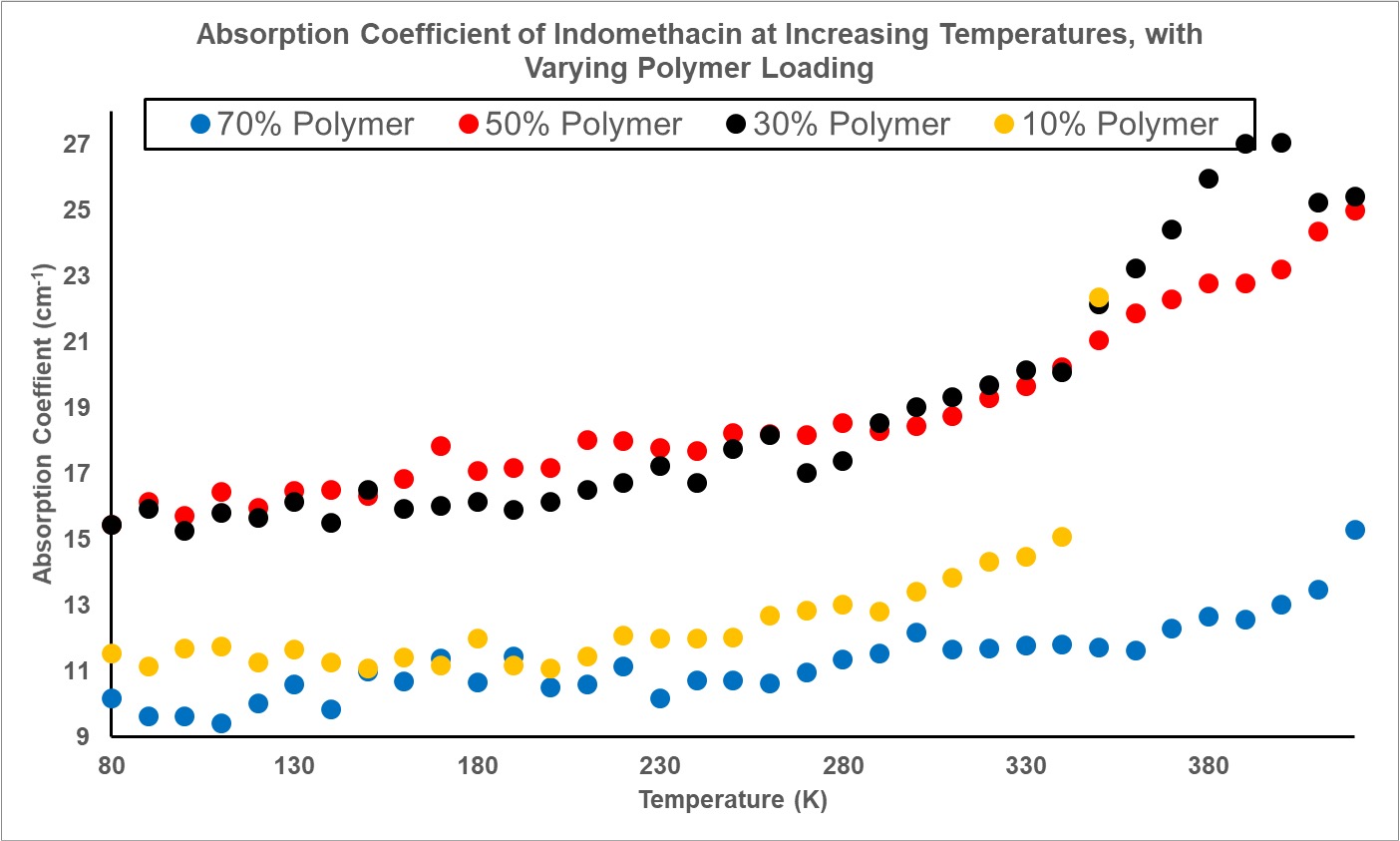 Figure 3. Absorption coefficient of indomethacin with varying polymer loading, analysed at 1 THz with increasing temperature.
Figure 3. Absorption coefficient of indomethacin with varying polymer loading, analysed at 1 THz with increasing temperature.