Formulation and Delivery - Biomolecular
Category: Late Breaking Poster Abstract
(M1530-12-78) Hot and Cold Formulation of Biologics for the Optimization of Drying Technologies
Monday, October 23, 2023
3:30 PM - 4:30 PM ET
- PL
Paulo Lino, Pharm.D.
Hovione PharmaScience SA
Lisbon, Lisboa, Portugal - SF
Susana Farinha, M.S.
Hovione PharmaScience SA
Lisboa, Lisboa, Portugal - LM
Luís Marques, Ph.D.
Hovione PharmaScience SA
Lisbon, Lisboa, Portugal - MG
Marco Galésio, Ph.D.
Hovione PharmaScience SA
Lisbon, Lisboa, Portugal - JC
Joana Cristovao, Ph.D.
Hovione PharmaScience SA
Lisbon, Lisboa, Portugal - MR
Miguel Ângelo Rodrigues, Ph.D.
Instituto Superior Tecnico de Lisboa
Lisbon, Lisboa, Portugal
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Biological drugs are expected to expand their pharmaceutical pipeline, as they offer targeted treatment options for a wide range of diseases [1,2]. However, maintaining the biomolecules’ stability throughout process development is a known challenge [3,4]. Thus, providing meaningful understanding of these complex biomolecules through fast and reliable data becomes increasingly crucial, as it paves the way for the success of every biotherapeutic, namely proteins [3,4]. Herein, the impact of different excipients was assessed during freeze drying (FD) and spray drying (SD) processes, using lysozyme as model drug. Insights into the formulations’ suitability for large-scale production and their ability to withstand the drying stresses commonly faced in the pharmaceutical industry was evaluated based on complementary data generated orthogonally by differential scanning fluorimetry (DSF), dynamic light scattering (DLS).
Methods: DSF with SYPRO Orange dye was used to identify excipients that either stabilized or destabilized the proteins by monitoring fluorescence shifts in the melting temperature (Tm). DLS was used to determine the aggregation temperature (Tagg). Dry powders of different lysozyme formulations were produced with FD using a standard drying cycle and with SD coupled with a two-fluid nozzle. The dried particles were characterized by Karl Fisher and scanning electron microscopy (SEM). Protein stability after reconstitution was evaluated with and size exclusion chromatography (SEC) and protein activity was evaluated with a lysozyme activity kit.
Results: Different formulations of lysozyme were studied: different sugars, polyols, buffers and surfactants. It was observed that all sugars and polyols stabilize lysozyme as denoted by the increase in lysozyme’s Tm. However, the stabilizing effect was not observed for all tested amino acids. Regarding buffers, it was noted that the buffer species and pH did play a significant role in protein stabilization, however the tested concentration range did not have a significant impact on Tm. Surfactants stabilizer function was established by DLS. All surfactants tested increased lysozyme’s Tagg, except for polysorbate 80 (PS80). Protein isolation was conducted using FD and SD for different formulations. FD was used to compare trehalose and mannitol as bulking agents, as well as to evaluate the influence of mannitol concentration. Additionally, the combinations of mannitol with arginine and glutamine, which exhibited stabilizing and destabilizing effects on lysozyme during formulation screening, were also evaluated. Two additional trials were conducted to assess the impact of mannitol combined with poloxamer 188 (P188) and to investigate the drying of lysozyme without any excipients. For spray drying, two trials were conducted: one using mannitol alone and the other using the combination of mannitol and P188 to evaluate the protective effect of P188 against shear stress during atomization. In FD trials, no significant differences were observed in particles’ morphology and the colloidal stability data was aligned with the formulation screening. A slight decrease in lysozyme’s colloidal stability after FD was observed for trehalose and mannitol formulations, as well as for the combination of mannitol with glutamine and P188. However, the combination of arginine with mannitol as well as the formulation without excipients significantly reduced lysozyme's colloidal stability after FD. The enzymatic activity results were consistent with colloidal stability data, except for the trial with P188. Despite maintaining colloidal stability, P188 had negatively impacted enzymatic activity, which was possibly related to its local concentration due to slow freezing. Conversely, SD rendered small spherical particles with controlled size and morphology while producing a negligible effect on lysozyme’s colloidal stability. Furthermore, for the same formulation, SD induced less protein degradation when compared to FD, which validates this technology as a mild process for biologics. Notably, under the tested processing conditions, the presence of P188 in the formulations had no impact on lysozyme's colloidal stability possibly because, other components, as mannitol are the main drivers for the protein stabilization during processing. To assess P188's effect, more aggressive atomization conditions could have been explored. Nevertheless, both formulations maintained lysozyme’s activity unchanged after SD, in contrast to the formulation with P188 in FD. This supports the hypothesis that processing technology conditions can specifically affect excipients, potentially compromising the biomolecule's critical quality attributes which is aligned with literature reports.
Conclusion: This work allowed to understand the interplay of a wide range of excipients in keeping protein activity and stability while fine tuning process conditions, forecasting scalable drug product development. In fact, in the case of P188, it showed compatibility issues with FD which ultimately impacted protein bioactivity. By relying on orthogonal techniques, protein:excipient interplays were studied to support excipient selection and improve the understanding of these complex systems during formulation design.
References: 1 Muralidhara, B. K. et al., Drug Discov. Today, 2020, 25, 574–581.
2 Mullard, A., Nat. Rev. Drug Discov., 2021, 20, 491–495.
3 Capelle, M. A. H. et al., Eur. J. Pharm. Biopharm., 2007, 65, 131–148.
4 Challener, C. A., Pharm. Technol., 2015, 39, 26–33.
Acknowledgements: This work was funded by Hovione under the doctoral fellowship PBID/BDE/11
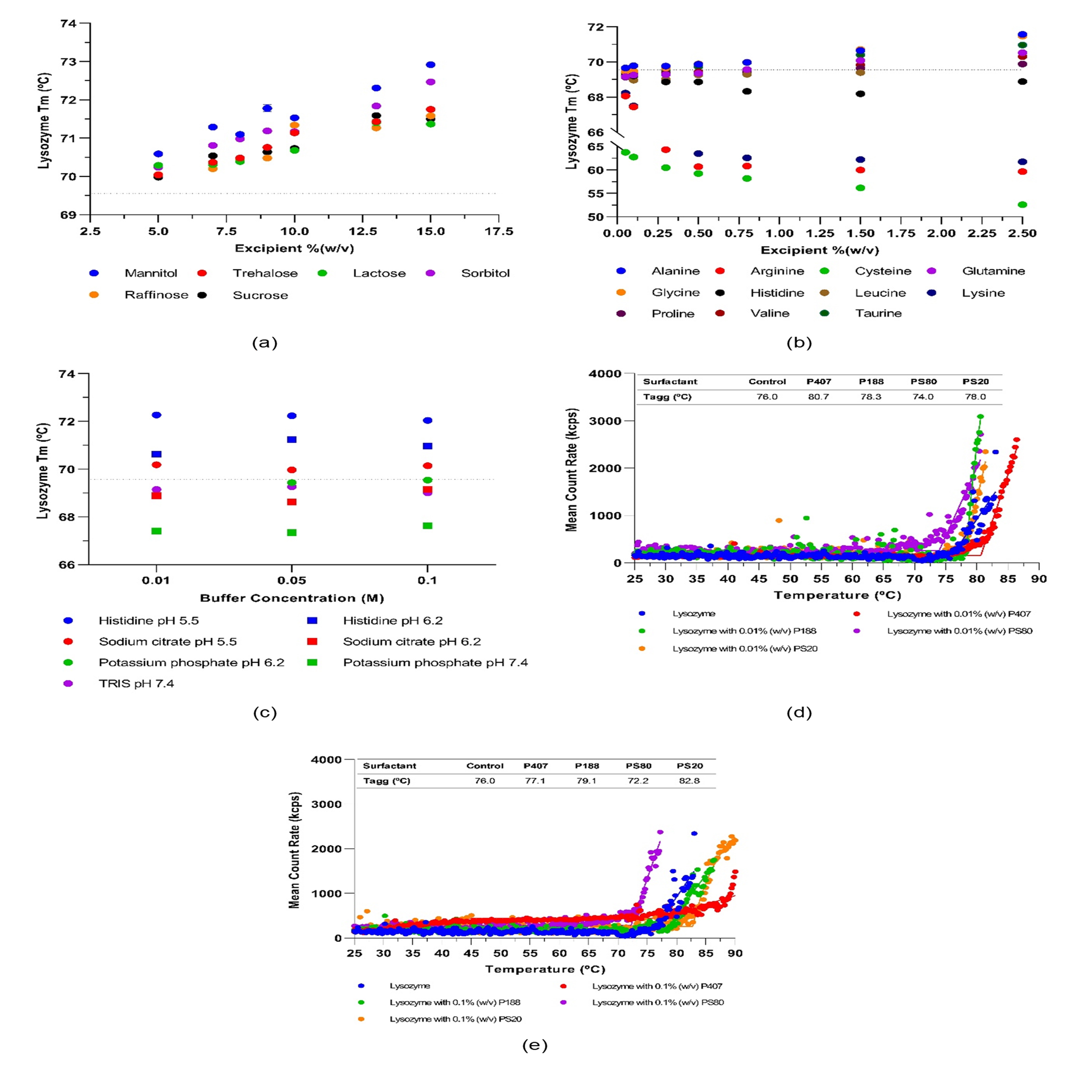 Melting temperatures of lysozyme in PBS buffer for different (a) sugars and polyols, (b) amino acids, and (c) buffers obtained with DSF and thermal ramps of lysozyme for different surfactants at (d) 0.01% (w/v) and (e) 0.1% (w/v) obtained with DLS.
Melting temperatures of lysozyme in PBS buffer for different (a) sugars and polyols, (b) amino acids, and (c) buffers obtained with DSF and thermal ramps of lysozyme for different surfactants at (d) 0.01% (w/v) and (e) 0.1% (w/v) obtained with DLS.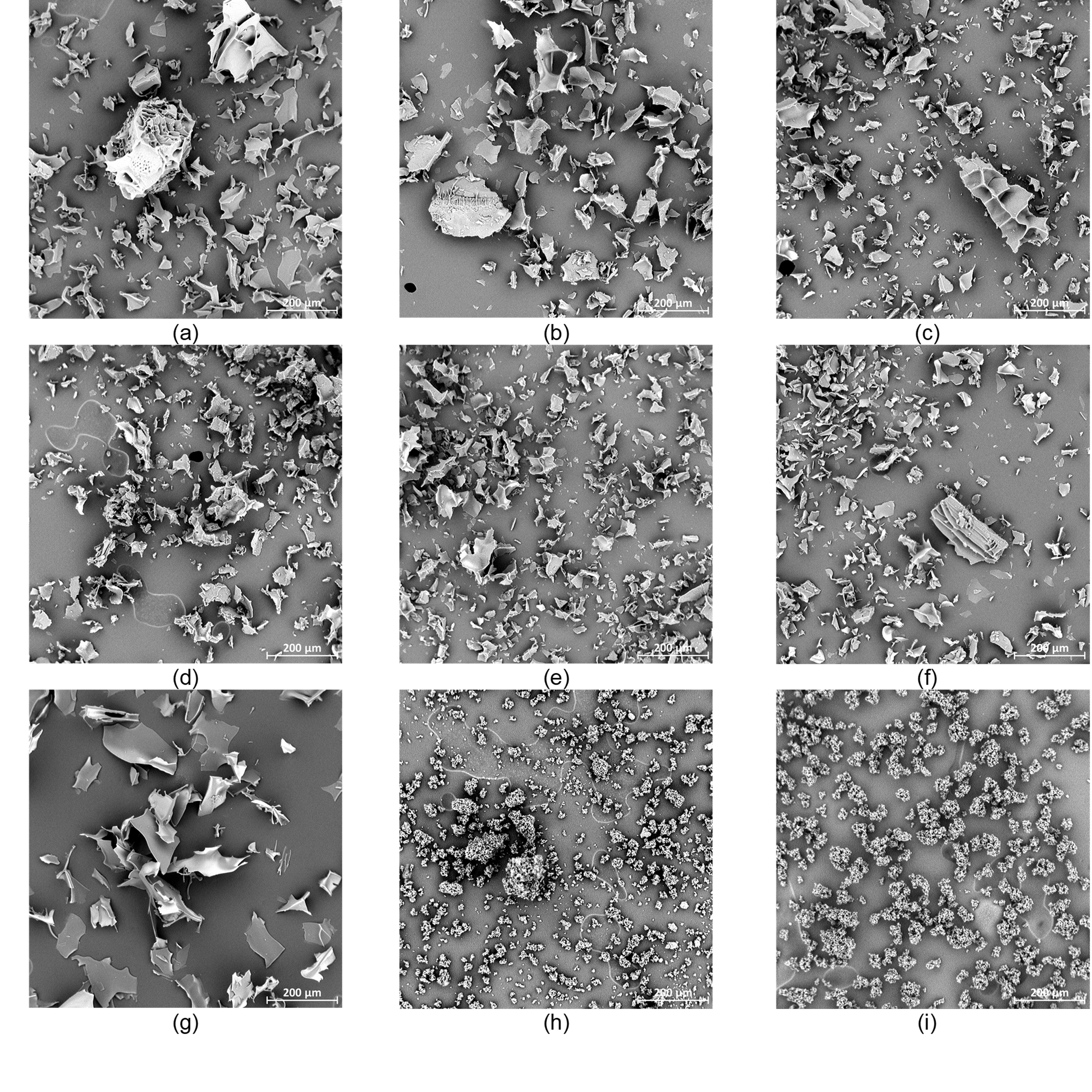 SEM images of freeze dried lysozyme formulations in 0.05 M Histidine pH 5.5 consisting of lysozyme with (a) 5% (w/v) trehalose, (b) 5% (w/v) mannitol, (c) 10% (w/v) mannitol, (d) 5% (w/v) mannitol and 2.5% (w/v) arginine, (e) 5% (w/v) mannitol and 2.5% (w/v) glutamine, and (f) 5% (w/v) mannitol and 0.01% (w/v) poloxamer 188 and (g) 2%(w/v) of lysozyme as well as spray dried formulations consisting of lysozyme with (h) 5% (w/v) mannitol and (i) 5% (w/v) mannitol and 0.01% (w/v) poloxamer 188.
SEM images of freeze dried lysozyme formulations in 0.05 M Histidine pH 5.5 consisting of lysozyme with (a) 5% (w/v) trehalose, (b) 5% (w/v) mannitol, (c) 10% (w/v) mannitol, (d) 5% (w/v) mannitol and 2.5% (w/v) arginine, (e) 5% (w/v) mannitol and 2.5% (w/v) glutamine, and (f) 5% (w/v) mannitol and 0.01% (w/v) poloxamer 188 and (g) 2%(w/v) of lysozyme as well as spray dried formulations consisting of lysozyme with (h) 5% (w/v) mannitol and (i) 5% (w/v) mannitol and 0.01% (w/v) poloxamer 188. 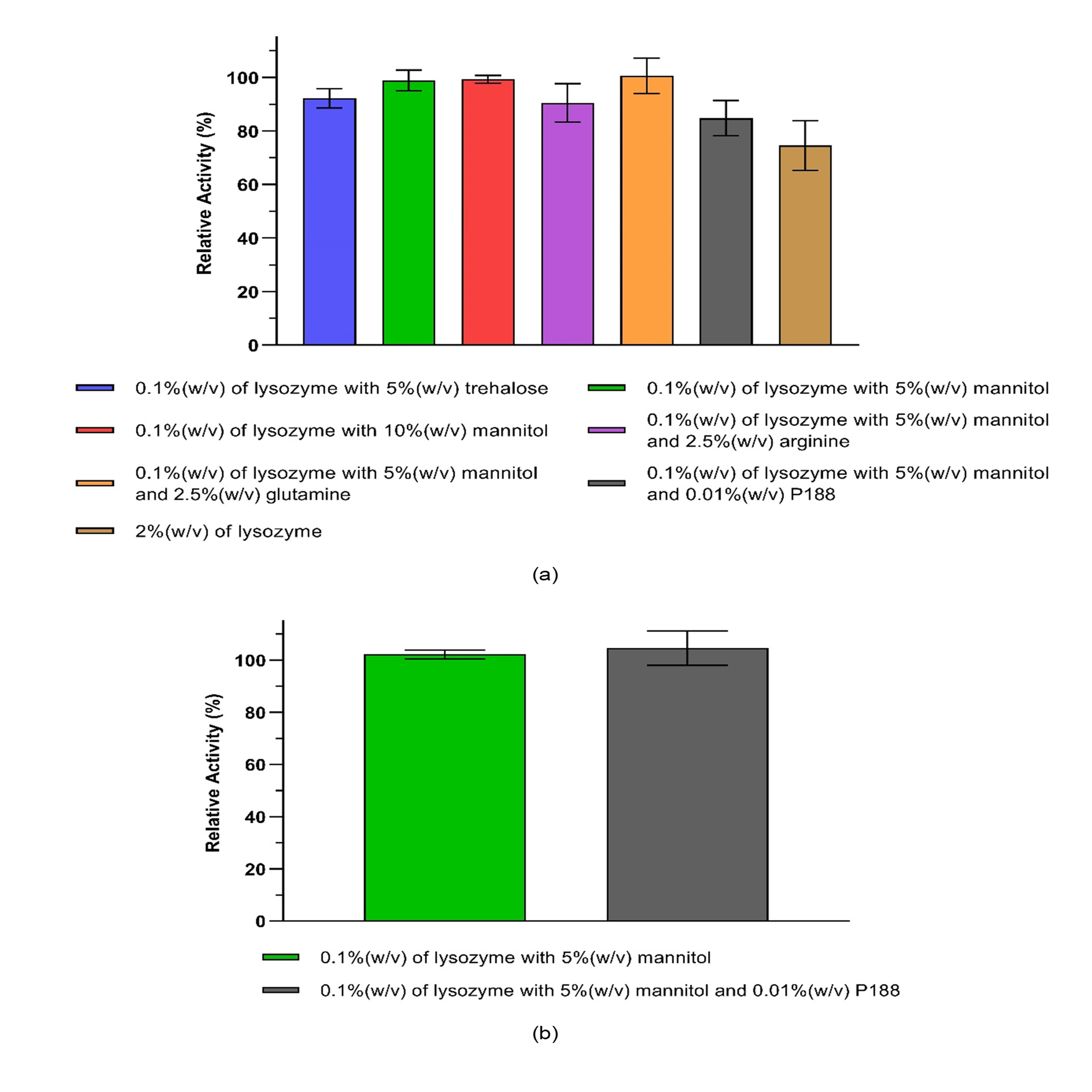 Relative activity for the different lysozyme (a) freeze dried and (b) spray dried formulations in 0.05 M Histidine pH 5.5.
Relative activity for the different lysozyme (a) freeze dried and (b) spray dried formulations in 0.05 M Histidine pH 5.5.