Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(M1530-09-60) Doxorubicin HCl Release from Liposomal Doxorubicin Formulations – Autonomous Capillary Electrophoretic (CE) In Vitro Release Test (IVRT) Method
Monday, October 23, 2023
3:30 PM - 4:30 PM ET
- SJ
Savithra Jayaraj, PhD (she/her/hers)
Research Fellow
US Food and Drug Administration
Jefferson, Arkansas, United States - SJ
Savithra Jayaraj, PhD (she/her/hers)
Research Fellow
US Food and Drug Administration
Jefferson, Arkansas, United States - WJ
Wenlei Jiang, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Thilak Mudalige, Ph.D. (he/him/his)
Research Chemist
US Food and Drug Administration
Jefferson, Arkansas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: A considerable amount of research has been conducted over the past six decades on the use of liposomes as drug delivery systems. In vitro drug release test (IVRT) is a critical quality control method in both premarket and post-approval regulation of liposomal drug products. Most IVRTs for liposomes require a separation step such as dialysis or solid phase extraction to quantitate released active pharmaceutical ingredient (API) without interference from the liposome bound API. However, these separation methods are lengthy and may cause an artificial drug concentration gradient or liposome rupture, resulting in inaccurate quantitation of released drug. The objective of the current work was to develop a new IVRT method to acquire accurate real-time release of doxorubicin from liposomal encapsulated doxorubicin hydrochloride formulations without additional separation steps.
Methods: We developed an automated capillary electrophoresis (CE) based method to determine the release of API from liposomal doxorubicin formulations. CE experiments were carried out on fused silica capillary and doxorubicin was detected using a high sensitivity UV-VIS detection cell. The capillary was conditioned with the background electrolyte solution (BGE) consisting of sucrose, polyethylene glycol (PEG) and phosphate prior to analysis. The release study was conducted at different temperatures (370C, 470C and 520C) and pH (5.5, 6.0, 6.5 and 7.4) conditions in release media containing histidine, ammonium formate, and sucrose. A sample (500 µL) was taken for CE analysis. The in-vitro drug release was automated, and the data were collected for 24 hours in 45 mins intervals continuously.
Results: The automated CE-based IVRT method can elute liposomal doxorubicin and released free doxorubicin at different times and provide quantitative measurements without additional sample preparation. The CE method was validated according to the U.S. FDA guidelines for bioanalytical method validation. A linear calibration was obtained by fitting the data with the linear regression model and the correlation coefficient (R2) calculated were greater than 0.998. The limit of quantification (LOQ) and limit of detection (LOD) for doxorubicin HCl were calculated to be 9.68 µM and 3.19 µM, respectively. The medium pH effect was investigated at 47°C ± 0.5°C and different pH conditions to mimic the pH at cancer cell (pH 5.5), cancer tissues (pH 6.5), and blood (pH 7.4). Based on release profiles obtained for reference standard, doxorubicin formulation manufactured by Sun Pharmaceuticals, the doxorubicin release rate increases with the increase of pH in the medium (Figure 1). The effect of temperature on the drug release of doxorubicin HCl was evaluated at 37°C, 47°C and 52°C for the generic formulation manufactured by Sun Pharmaceuticals. It was observed that the drug release increased with the increase of media temperature (Figure 2). Complete doxorubicin release (100%) was obtained in 7 hours at pH 6.5 and 47°C, and in less than 3 hours at pH 6.5 and 52°C conditions. The drug release profiles obtained for the five liposomal doxorubicin formulations manufactured by Baxter Healthcare Corporation (Baxter), Sun Pharmaceutical Industries Ltd (Sun Pharma), Dr. Reddy’s Laboratories Ltd (Dr. Reddy), Ayanna Pharma Ltd (Ayana Pharma), and Zydus Pharmaceuticals (Zydus), were similar at pH 6.5 and 47ºC (Figure 3).
Conclusion: We developed a new CE-based approach that delivers a better fully automated IVRT method for liposomal formulations that uses a small amount of release samples, continuous sampling, and no additional separation step for the API. This method was demonstrated for liposomal formulations containing doxorubicin hydrochloride. This CE-based method can be applied for in vitro release profiling of other liposome drug products.
Acknowledgements: These studies were conducted using the Nanotechnology Core Facility (NanoCore) located on the U.S. Food and Drug Administration’s Jefferson Laboratories campus (Jefferson, AR) with collaborations among ORA/ORS and CDER/OGD/ORS. Dr. Savithra Jayaraj was supported in part by an appointment to the Research Participation Program at the U.S. Food and Drug Administration administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. The opinions expressed in this abstract are those of the authors. The opinions should not be interpreted as current or future policy of the U.S. Food & Drug Administration or any other agency of the U.S. government. The mention of manufacturers or trade names are for experimental clarity and does not constitute product endorsement.
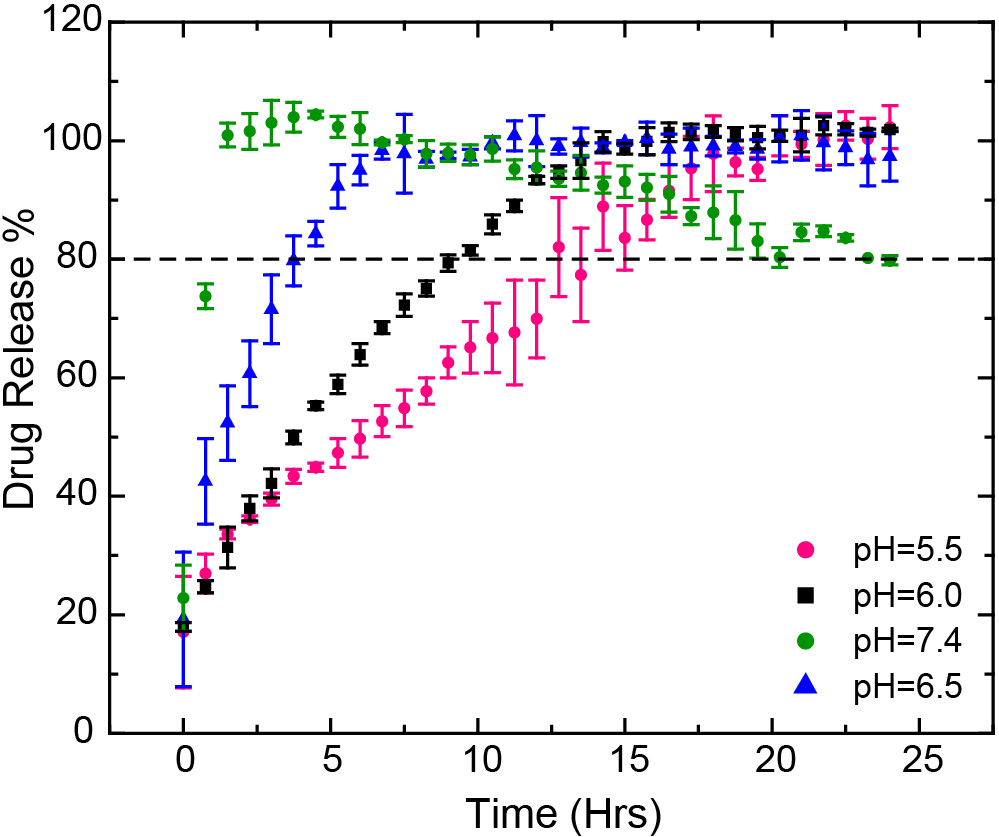 Figure 1. Drug release profiles of the Sun Pharma formulation of the liposomal doxorubicin HCl at 47ºC and different pH release mediums (pH 5.5, pH 6.0, pH 6.5 and pH 7.4) (mean ± SD, N=3).
Figure 1. Drug release profiles of the Sun Pharma formulation of the liposomal doxorubicin HCl at 47ºC and different pH release mediums (pH 5.5, pH 6.0, pH 6.5 and pH 7.4) (mean ± SD, N=3).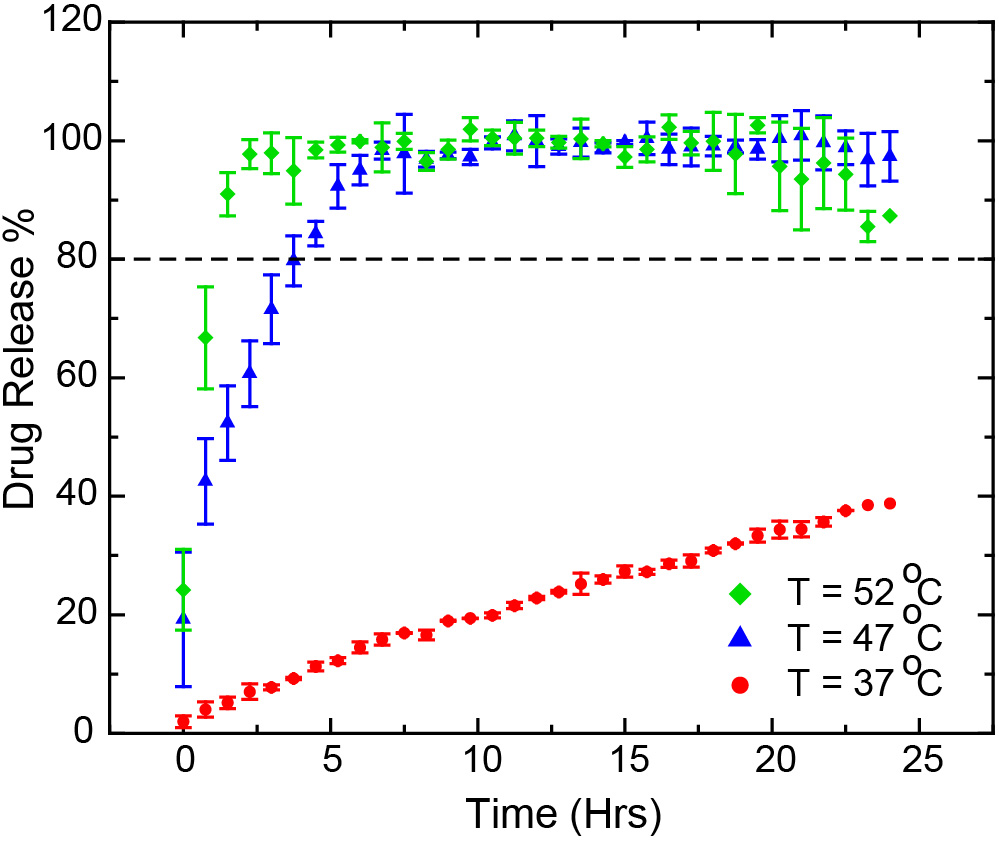 Figure 2. Drug release profiles of the Sun Pharma formulation of the liposomal doxorubicin HCl in pH 6.5 medium and different temperatures (37°C, 47°C and 52°C) (mean ± SD, N=3).
Figure 2. Drug release profiles of the Sun Pharma formulation of the liposomal doxorubicin HCl in pH 6.5 medium and different temperatures (37°C, 47°C and 52°C) (mean ± SD, N=3).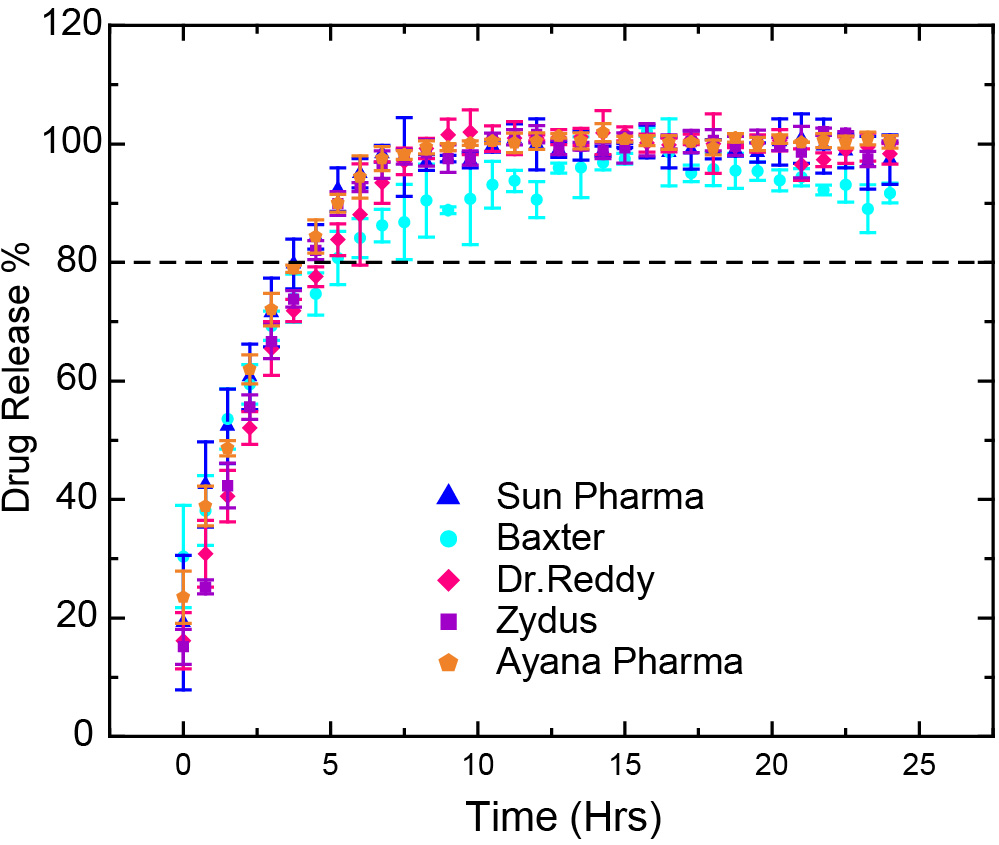 Figure 3. Drug release profiles of five different formulations of the liposomal doxorubicin HCl at pH 6.5 and 47ºC (mean ± SD, N=3).
Figure 3. Drug release profiles of five different formulations of the liposomal doxorubicin HCl at pH 6.5 and 47ºC (mean ± SD, N=3).