Discovery and Basic Research
Category: Poster Abstract
(M0930-04-25) A Compatibility Test of Two-Piece Hard Capsules and Liquid/Semi-solid Excipients - Part 2
Monday, October 23, 2023
9:30 AM - 10:30 AM ET

Mamoru Honda, BA (he/him/his)
Deputy manager
Qualicaps Co., Ltd.
Souraku-gun, Kyoto, Japan- NK
Nobuhiro Kato, BA
Qualicaps Co., Ltd.
Souraku-gun, Kyoto, Japan
Presenting Author(s)
Main Author(s)
Purpose: Many of the new drug candidate compounds developed in recent years are said to be poorly water-soluble. Various studies have been conducted to improve the absorption and solubility of these compounds, and liquid formulation is one of the methods. Liquid/semi-solid based dosage forms enhance bioavailability and solve problems caused by raw material properties such as poor water solubility, low dosage, high potency, low melting point, or critical stability profiles. Two-piece hard capsules are a suitable solid oral dosage form for liquid filling. However, their use for this application has not been sufficiently studied. The purpose of this study is to provide data on the compatibility of three types of hard two-piece capsules and various liquid/semi-solid excipients by means of stability test at accelerated environmental conditions. In the presentation of Part 1, an accelerated test was performed by putting the capsules in a glass bottle with a polypropylene cap, but the Polypropylene cap permeated water vapor, so the humidity in the container was not stable. This time, we performed an accelerated test by putting the capsules in an aluminum vapor deposition bag that does not allow water vapor to pass through. We also think the humidity when the capsules are filled is important for the results of stability data, so we also strictly control humidity during the filling with liquid/semi-solid excipients.
Methods: Three types of hard capsules were tested: gelatin capsule (QUALI-GTM), PEG/gelatin capsule (QUALI-GTM-PEG) and hypromellose capsule (Quali-V®). For the filling, we chose 20 excipients that are used in liquid formulations, such as vegetable oil and surfactants. The capsules were band-sealed with their own formulation material after filling in order to prevent possible leakage in the case of low viscosity liquid. The capsules were placed in aluminum vapor deposition bag and stored under the conditions at 40℃ and 75%RH, and the appearance and disintegration properties were evaluated after 6months. The disintegration test was performed according to the method described in the USP using water as the test medium, and it was confirmed whether the contents were released after 20 minutes. The disintegration test was performed only on samples with no problem in appearance.
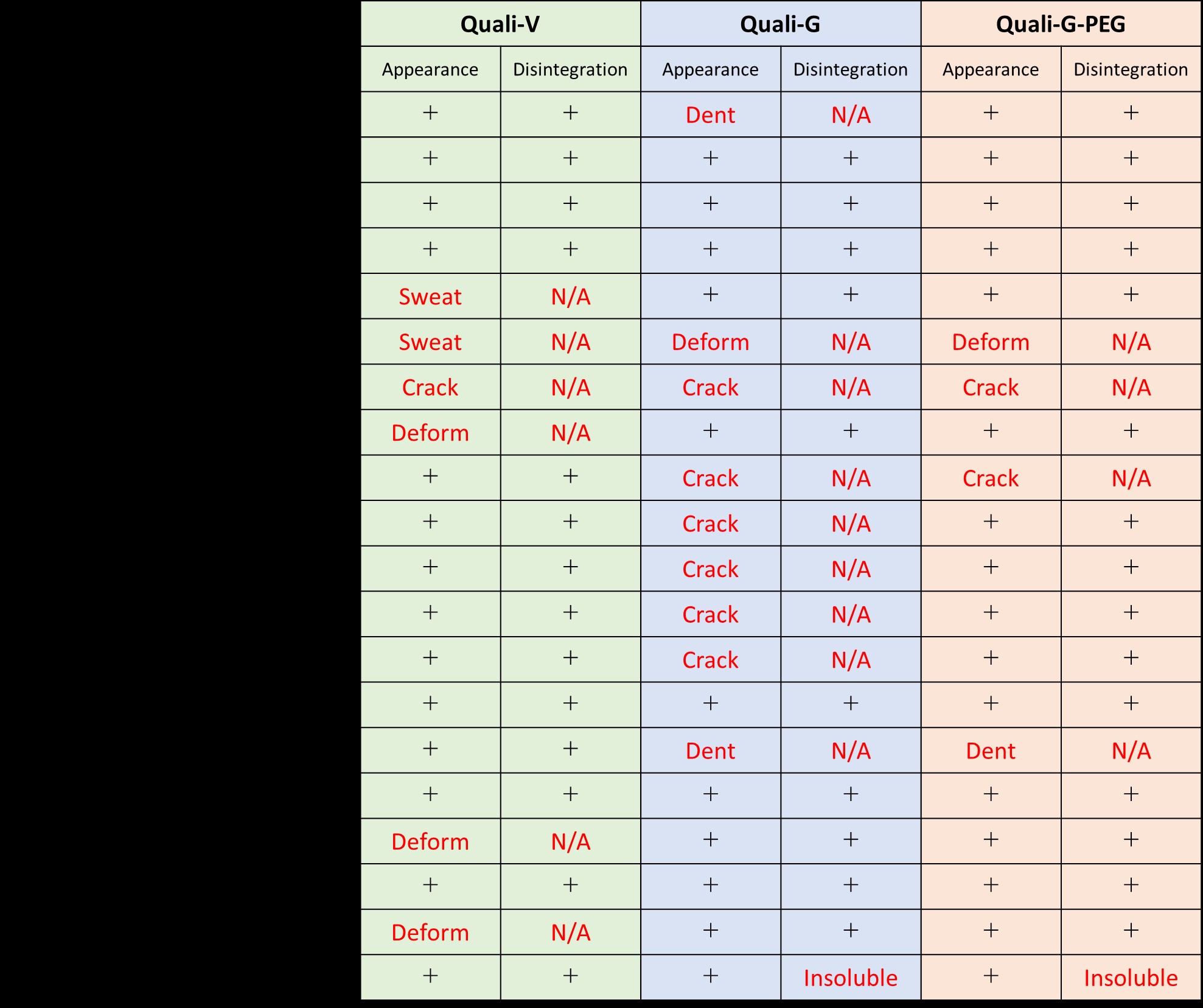
Results: Most of the samples had no problem in appearance and disintegration test after the accelerated test. 14 samples of Quali-V, 10 samples of Quali-G, and 15 samples of Quali-G-PEG were compatible. However some samples were incompatible.
Appearance
Four types of defects were observed (Table 1).
1. Deform: capsule deforms and the contents leak out.
2. Dent: capsule deforms, but the contents do not leak out.
3. Sweat: capsule does not deform, but the contents leak out.
4. Crack: capsule cracks and the contents leak out.
Glycerol and Diethylene glycol monoethyl ethers were not compatible with all capsules.
Quali-V filled with PEG400, Caprylocaproyl polyoxyl-8 glycerides or Propylene glycol monocaprylate were deformed.
Quali-V filled with Lauric acid sweated.
Quali-G and Quali-G-PEG filled with Sorbitan trioleate were heavily dented.
Quali-G cracked with some excipients, but Quali-G-PEG, which contains PEG as a plasticizer, cracked less.
Disintegration test
ALL Quali-V samples completely released their contents.
Quali-G and Quali-G-PEG filled with Polyoxyl-40 stearate were insolubilized.
Quali-G and Quali-G-PEG sometimes left an insoluble thin film (pellicle) due to gelatin cross-linking, but the contents were released.
By storing the capsules filled with excipients in an aluminum vapor deposition bag, the number of compatible samples increased compared to the results for glass bottle with polypropylene caps presented in Part1.
Conclusion: The compatibility of three types of hard capsule and liquid/semi-solid excipients was evaluated. There were a few liquids excipients that were not compatible with all capsules, but it was found that a wide range of liquid/semi-solid excipients could be filled by choosing the suitable capsules for each excipients. From this result, it was found that hard capsules can be filled various liquid/semi-solid excipients.