Formulation and Delivery - Biomolecular
Category: Poster Abstract
BIONDD<sup>TM</sup> Oral Capsule Delivery of Liraglutide with Bioavailability Comparable to Subcutaneous Injection and Development of Efficient In Vivo Model in Dogs to Test Feasibility of Other Biologic Drugs
(T1530-02-08) BIONDDTM Oral Capsule Delivery of Liraglutide with Bioavailability Comparable to Subcutaneous Injection and Development of Efficient In Vivo Model in Dogs to Test Feasibility of Other Biologic Drugs
Tuesday, October 24, 2023
3:30 PM - 4:30 PM ET

Nina Mertz, PhD (she/her/hers)
Industrial Postdoc
Biograil ApS
Roskilde, Sjelland, Denmark
Nina Mertz, PhD (she/her/hers)
Industrial Postdoc
Biograil ApS
Roskilde, Sjelland, Denmark- NS
Nikolaj Skak, MS (he/him/his)
Biograil ApS
Roskilde, Sjelland, Denmark - KL
Karsten Lindhardt, Ph.D. (he/him/his)
Biograil ApS
Roskilde, Sjelland, Denmark
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Oral delivery is by far the most preferred administration route for pharmaceuticals, but limited absorption of biologics such as peptides, proteins, RNAs, and antibodies has been a major challenge [1, 2, 3]. A new group of novel technologies delivering active substances into the wall instead of in the lumen of the GI tract lumen has shown promising results with relative bioavailabilities comparable to subcutaneous (SC) injection [1, 2, 3]. The BIONDDTM capsule is swallowed and, when exposed to liquids in the stomach, a mechanism for the insertion of active substances into the stomach wall is triggered. This allows for the effective distribution of biomacromolecules to the bloodstream and circulation which today would need to be injected. The BIONDDTM technology fits into a conventional size 0 capsule giving it an attractive small size for effective and convenient oral administration. The feasibility of the BIONDDTM capsule for delivery of a biologic through intra-wall (IW) administration is investigated using a four-step approach as described in Figure 1. First, the exposures to the biologic in question after SC injection (step 1) and IW injection (step 2) are compared to confirm that systemic delivery can be achieved via intra-wall administration. Next, it is explored if comparable delivery can be observed after IW insertion of a solid formulation (step 3) before the delivery of the biologic following oral administration of the BIONDDTM capsule (step 4) is investigated. The purpose of the current work was to investigate the feasibility of the BIONDDTM technology for oral delivery of liraglutide in vivo in dogs utilizing the four-step approach (Figure 1).
Methods: 0.4 mg/mL liraglutide solutions for SC injection and intra-wall injection were prepared by dilution of Victoza® (Novo Nordisk, Bagsvaerd, Denmark) or a liraglutide stock solution, respectively, into 0.9% saline solution. 40%w/w liraglutide (Chengdu Shengnuo Biopharm Co., Chengdu, China) mixed with 60 %w/w Poly(ethylene glycol) 6000 (Ph Eur, Sigma-Aldrich, Missouri, USA) at 67⁰C were loaded into the BIONDDTM spikes. In the four-step in vivo study (Figure 1) male beagle dogs received a liraglutide dose of 0.4 mg/dog administered as a SC injection (N=6), intra-wall injection (N=3), intra-wall spike insertion (N=3), or BIONDDTM capsule (N=9). Prior to dosing the dogs were fasted overnight. The dogs received 40 mL of water by oral gavage tube before placement of the BIONDDTM capsule on the base of the tung. The capsule was swallowed by administration of 10 mL of water. The successful insertion of the BIONDDTM spike was evaluated by gastroscopic inspection 1 h after dosing. The dog plasma liraglutide concentration was determined using a customized ELISA kit (BioAgilytix, Hamburg, Germany). The housing of the animals was in accordance with EU Directive 2012/63/EU of 22 September 2012 on the protection of animals for scientific purposes and the animal experimentation was carried out under a license provided by the National Animal Experiments Inspectorate under the Ministry of Food, Agriculture and Fisheries of Denmark.
Results: The four-step approach (Figure 1) was successfully used to evaluate the exposure to liraglutide following SC or intra-wall (IW) injection of a liquid formulation (step 1 and step 2) and IW spike insertion or oral BIONDD capsule dosing of a solid formulation (step 3 and step 4). The obtained pharmacokinetic (PK) profiles show that comparable exposure to liraglutide was observed after a dose of 0.4 mg/dog was administered in the four steps (Figure 2). Relative to SC injection 93% bioavailability was observed after IW injection (Figure 2, Table 1) proving IW administration to be a feasible route of administration for liraglutide. A PK profile comparable to those seen after injection of a liquid formulation (steps 1 and 2) was observed after IW spike insertion (step 3) indicating that similar exposure to liraglutide was achieved with the solid formulation (Figure 2). In step 4, oral dosing with the BIONDDTM capsule resulted in 116% bioavailability (relative to SC injection of Victoza®). Tmax and Cmax values were in the same range as observed for SC administration (Table 1).
Conclusion: The results presented show that convenient oral administration of the attractive small-size BIONDDTM capsule technology provides highly effective systemic drug delivery of the biologic drug liraglutide. Comparable exposure of liraglutide was obtained after oral dosing of the BIONDDTM capsule and SC injection indicating that this stomach intra-wall delivery technology is very promising. A four-step approach to evaluate the feasibility of drugs for oral dosing with the BIONDDTM technology for efficient selection of relevant compounds for the technology was developed. The consistent data indicate the model is relevant and will provide an understanding of IW delivery feasibility of various drugs to be tested in the future. The BIONDD TM technology is believed to have the potential to transform injectable pharmaceutical products into convenient oral capsules.
References: [1] Abramson et al. An ingestible self-orienting system for oral delivery of macromolecules. Science. 2019 Feb 8;363(6427):611-615
[2] Abramson et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat Med. 2019 Oct;25(10):1512-1518
[3] Traverso et al. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci. 2015 Feb;104(2):362-7
Acknowledgements: Scantox (Lille Skensved, Denmark) is acknowledged in their support to develop the four-step in vivo model and IW precision delivery with the gastroscope (Step 2 and Step 3).
Funding:
Biograil ApS has received co-funding for a 2-year Industrial Postdoc project from Innovation Fund Denmark.
Disclosures: Karsten Lindhardt and Nikolaj Skak are founders of Biograil Aps a pre-clinical stage pharmaceutical company developing the BIONDDTM technology for oral delivery of biologics. Nina Mertz is employed as an Industrial Postdoc at Biograil ApS.
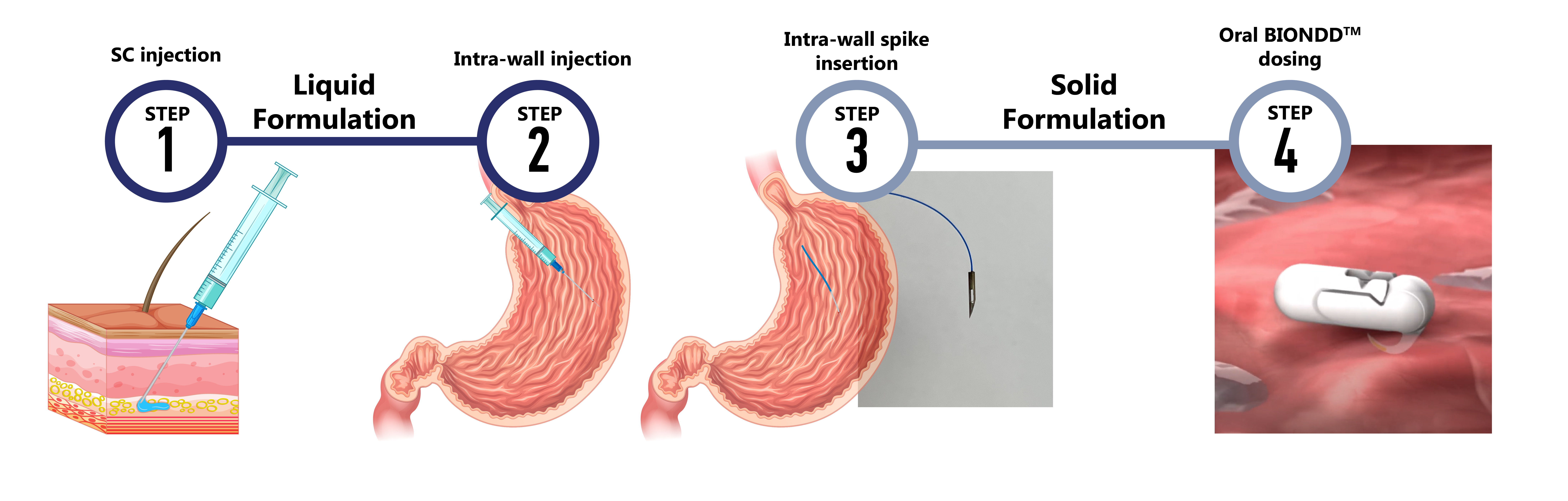 Figure 1: The 4-step approach for the in vivo feasibility testing of intra-wall delivery of a biologic in dogs with the BIONDDTM capsule. Step 1: Subcutaneous (SC) injection of a liquid formulation. Step 2: Intra-wall injection of a liquid formulation into the stomach wall performed using a gastroscope. Step 3: Intra-wall insertion of a BIONDDTM spike loaded with solid formulation into the stomach wall using a gastroscope. Step 4: Oral administration of the BIONDDTM capsule loaded with a solid formulation.
Figure 1: The 4-step approach for the in vivo feasibility testing of intra-wall delivery of a biologic in dogs with the BIONDDTM capsule. Step 1: Subcutaneous (SC) injection of a liquid formulation. Step 2: Intra-wall injection of a liquid formulation into the stomach wall performed using a gastroscope. Step 3: Intra-wall insertion of a BIONDDTM spike loaded with solid formulation into the stomach wall using a gastroscope. Step 4: Oral administration of the BIONDDTM capsule loaded with a solid formulation.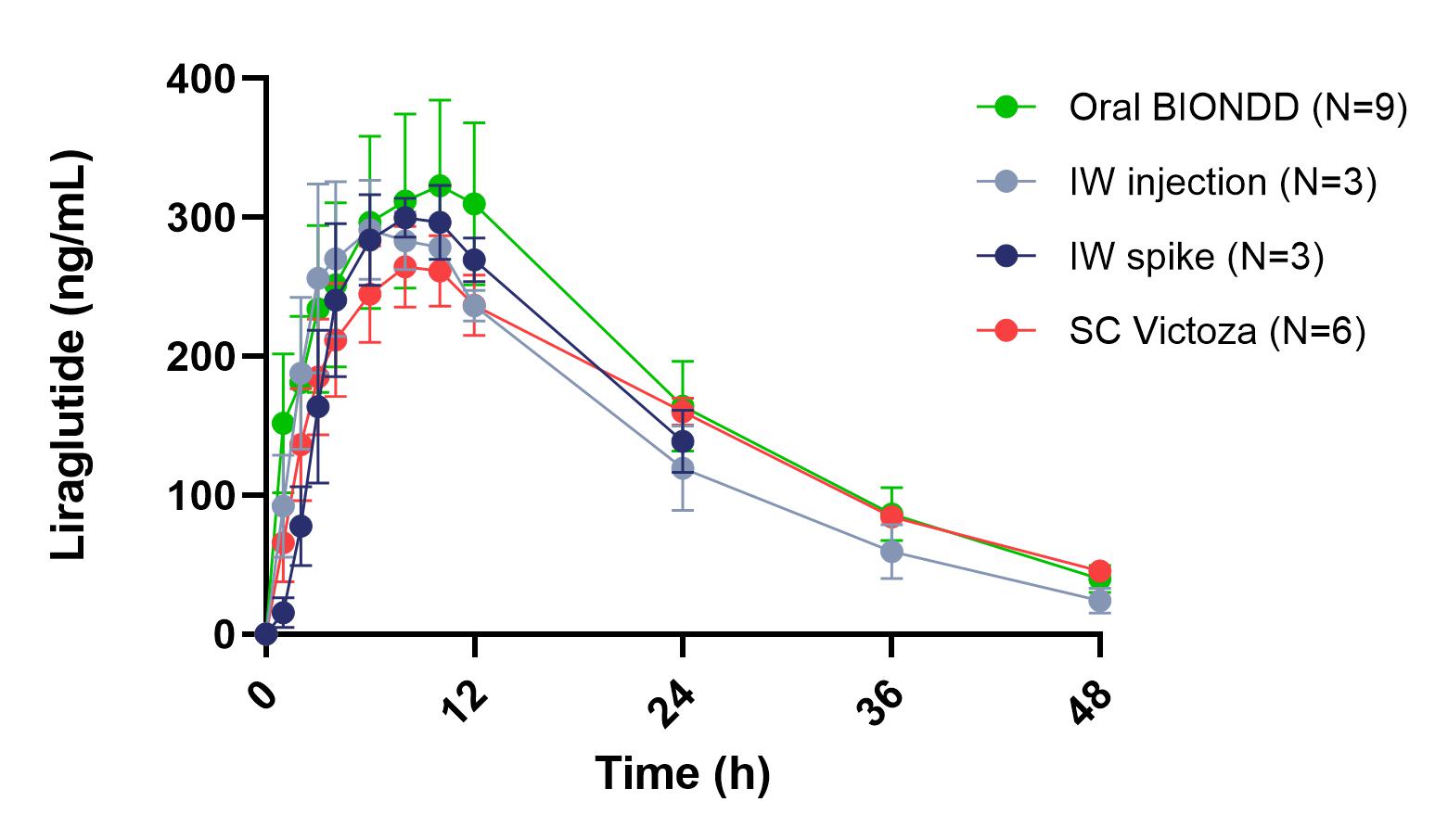 Figure 2: Pharmacokinetics of liraglutide in beagle dogs. The time-course change in plasma concentrations of liraglutide delivered by subcutaneous (SC) or intra-wall (IW) injection of Victoza® or a liraglutide solution, respectively, and by IW spike insertion and oral administration of the BIONDDTM capsule (liraglutide dose: 0.4 mg/dog) is shown. Data are presented as mean ± SEM.
Figure 2: Pharmacokinetics of liraglutide in beagle dogs. The time-course change in plasma concentrations of liraglutide delivered by subcutaneous (SC) or intra-wall (IW) injection of Victoza® or a liraglutide solution, respectively, and by IW spike insertion and oral administration of the BIONDDTM capsule (liraglutide dose: 0.4 mg/dog) is shown. Data are presented as mean ± SEM. 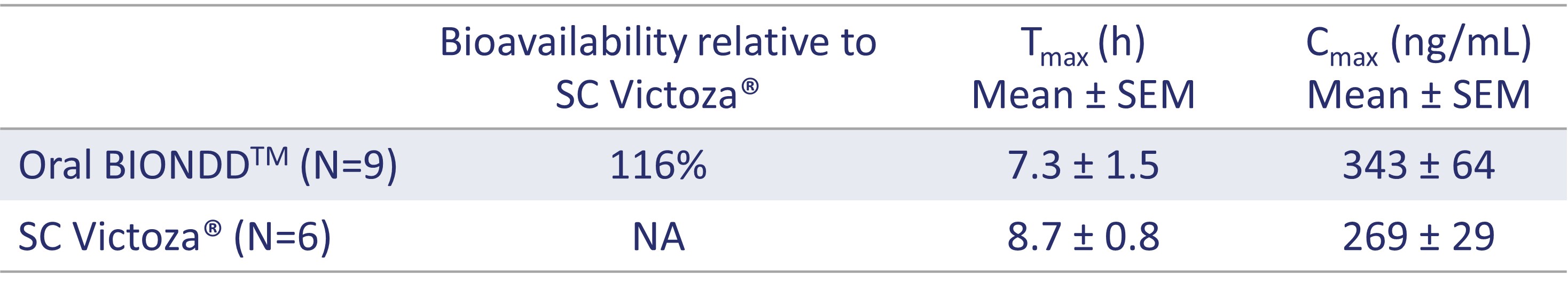 Table 1: Pharmacokinetic parameters for liraglutide administered by subcutaneous (SC) injection of Victoza® and orally with the BIONDDTM capsule (liraglutide dose: 0.4 mg/dog).
Table 1: Pharmacokinetic parameters for liraglutide administered by subcutaneous (SC) injection of Victoza® and orally with the BIONDDTM capsule (liraglutide dose: 0.4 mg/dog).