Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(T1530-08-54) Critical Formulation Attributes of Naloxone Nasal Spray Products Affecting Nasal Permeation

Ahmed S. Zidan, PhD
Senior Staff Fellow (Pharmacologist)
US Food and Drug Administration
Silver Spring, Maryland, United States
Ahmed S. Zidan, PhD
Senior Staff Fellow (Pharmacologist)
US Food and Drug Administration
Silver Spring, Maryland, United States- TS
Tasmin Ara Sultana (she/her/hers)
US Food and Drug Administration
Silver Spring, Maryland, United States - JQ
Juliana Quarterman, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - BN
Bryan Newman, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - MA
Manar Al-Ghabeish, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Steven Chopski, PhD (he/him/his)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States- RW
Ross Walenga, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - VP
Venkateswara Pavuluri, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Diaa Shakleya, PhD
Senior Research Scientist (Pharmacologist)
US Food and Drug Administration
Silver Spring, Maryland, United States- ML
Min Li, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - MA
Muhammad Ashraf, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Co-Author(s)
Purpose: Narcan® nasal spray is a solution formulation of Naloxone hydrochloride (NH) for the emergency treatment of opioid overdose. It may be easily and efficiently administered through nose in emergency to produce immediate response and save lives. The bioavailability of several NH nasal spray products, differing in formulation composition, e.g., type of preservative, buffer system, pH, etc., have been evaluated in clinical studies and reported in literature. In the current study, effects of various parameters of nasal administration and formulation variables on the nasal bioavailability of NH was investigated using physiologically based biopharmaceutics modeling (PBBM) analysis coupled with in vitro permeation testing (IVPT) of representative NH formulations.
Methods: The IVPT method for NH spray was developed using EpiAirwayTM mucociliary tissue model mounted to Ussing chamber to investigate the effects of preservative type (benzyl alcohol (BA) or benzalkonium chloride (BC)) on the permeability of NH solution (4 mg/mL). For drug dosing in IVPT runs, the Vitrocell cloud alpha system was used to deposit NH on EpiAirwayTM mucociliary tissues. Kreb’s buffer was used for the IVPT method and samples were collected over 4 hours. The in-vitro permeation pattern of NH deposited on artificial membranes (Nuclepore Track-Etch or Durapore membranes) was also investigated using flow through cells assembly. NH was deposited on the membranes in a Vitrocell cloud alpha system by nebulizing the NH nasal solution through a 4 to 6 micron vibrating mesh nebulizer for 40 seconds. Dulbecco’s Phosphate Buffered Saline fortified with Calcium and Magnesium was used as the receptor medium in flow through cells and was pumped at a flow rate of 50 uL/min. Permeation samples across the membranes were collected at predetermined time points over a period of 2 hours. The concentration of Naloxone in the samples was determined using a validated stability indicating LC/MS method.
Results: Our PBBM based analysis indicated that neither administration of NH spray to one versus two nostrils nor changing dose volume from 100 to 200 µL affected Cmax significantly (p > 0.05). The formulation pH significantly affected the Cmax (p ≤ 0.0015) and AUC0-inf (p < 0.0001). The results in Figure 1A showed that addition of BA and citric acid buffer in the formulation significantly decreased the Cmax compared to that of Narcan (p ≤ 0.013). The IVPT results in Figure 1B demonstrated that replacing BA with BC increased the permeability coefficient of NH. The permeation results in Figure 1C and 1D showed that Naloxone permeation across the membranes plateaued off after 20-45 minutes which may be due to drug depletion in the donor chamber. The results in Figure 1E showed that maximum flux of Naloxone was attained within 5 minutes of assembling the flow through cells. Similarly, the results in Figure 1F show that permeation rates were highest initially during IVPT experiment using EpiAirwayTM mucociliary tissue model in Kreb’s buffer.
Conclusion: This study concluded that the nasal bioavailability of NH may not be sensitive to variations in administration parameters. A biorelevant IVPT method may be helpful in identifying critical formulation parameters of NH nasal spray. The in vitro permeation characteristics of naloxone nasal spray products across may be used as a surrogate for assessing the availability of naloxone for nasal permeation. Further studies are ongoing to investigate the sensitivity of these in vitro permeation method to changes in formulation attributes of naloxone spray products.
Acknowledgements: Disclaimer: This abstract reflects the views of the authors and should not be construed to represent FDA’s views or policies.
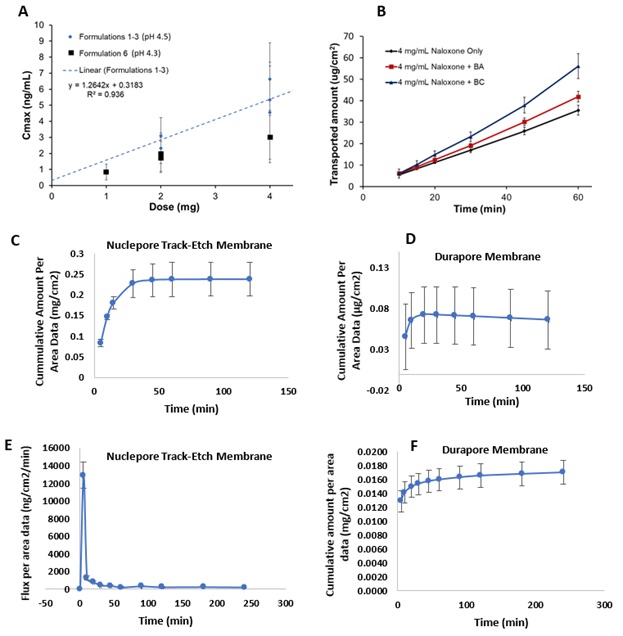 Figure 1. A) Effect of dose strength on Cmax following nasal administration of NH, and B) Effect of preservatives on the in-vitro permeation of NH nasal formulations across muco-ciliary tissue model. C) Cumulative Amount of Naloxone dissolved per surface area of diffusion (mg/cm2) in Nuclepore Track-Etch Membrane and D) Durapore Membrane. E) Flux and F) Cumulative amount of Naloxone dissolved and permeated in Kreb’s buffer through the EpiAirwayTM mucociliary tissue model as a function of time.
Figure 1. A) Effect of dose strength on Cmax following nasal administration of NH, and B) Effect of preservatives on the in-vitro permeation of NH nasal formulations across muco-ciliary tissue model. C) Cumulative Amount of Naloxone dissolved per surface area of diffusion (mg/cm2) in Nuclepore Track-Etch Membrane and D) Durapore Membrane. E) Flux and F) Cumulative amount of Naloxone dissolved and permeated in Kreb’s buffer through the EpiAirwayTM mucociliary tissue model as a function of time.