Formulation and Delivery - Chemical
Category: Poster Abstract
(T1530-11-73) Investigating the Relationship between Microstructural Properties and In Vitro Release Characteristics of PLGA Microspheres

Ruifeng Wang, MS (he/him/his)
Research Assistant
University of Connecticut
STORSS, Connecticut, United States
Ruifeng Wang, MS (he/him/his)
Research Assistant
University of Connecticut
STORSS, Connecticut, United States- AC
Andrew G. Clark, Ph.D. (he/him/his)
DigiM Solution LLC
Woburn, Massachusetts, United States - QB
Quanying Bao, Ph.D. (she/her/hers)
University of Connecticut
Storrs, Connecticut, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - BQ
Bin Qin, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Shawn Zhang, Ph.D. (he/him/his)
Managing Partner
DigiM Solution LLC
Woburn, Massachusetts, United States
Diane J. Burgess, Ph.D. (she/her/hers)
Distinguished Professor
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: When developing complex generic products, such as poly (lactic-co-glycolic acid) (PLGA) microspheres, qualitative (Q1) and quantitative (Q2) sameness are not enough to ensure the equivalence of the two products. It is also crucial to consider the similarity in microstructure (Q3), as this may affect drug release behavior and consequently, their efficacy and safety. The aim of this study is to employ focused ion beam scanning electron microscopy (FIB-SEM) combined with quantitative artificial intelligence (AI)-based image analytics to explore the relationship between microstructure and release characteristics of microspheres.
Methods: Minocycline hydrochloride was selected as the model drug. Four microsphere formulations were prepared via a coacervation method using a well-designed glass vessel assembly as previously described [1]. The physicochemical properties including drug loading, particle size, and morphology of the prepared microspheres were characterized. In vitro release testing of the microspheres was conducted using a sample-and-separate method. The internal microstructures of the different microsphere formulations were investigated through repeated FIB milling and subsequent SEM imaging (Figure 1A). The 3D microstructure properties including volume fraction, size distribution, and spatial distribution of all visible phases (polymer, drug, and pore) were quantified from the FIB-SEM image using AI image analytics (DigiM™ I2S cloud-based image analysis platform).
Results: Four compositionally equivalent formulations (FA, FB, FC, and FD) of minocycline hydrochloride microspheres were successfully prepared with different processing conditions. The drug loading of all four formulations was around 26% (Table 1). The four in-house formulations showed different in vitro drug release characteristics (Figure 1D). Compared to formulations FB and FD, formulations FA and FC showed lower release rates. Accordingly, more drug particles were observed inside the in vitro release samples of FA and FC on Day 6 of release (Figure 1E). All internal material phases, including drug particles, pores, and polymer, were identified and quantified successfully through FIB-SEM and AI image analytics (Figure 1B and C). The in vitro release rates were confirmed to be related to the porosity and material spatial distribution. The formulation FC with the lowest pore volume fraction showed the lowest release rate. The microspheres developed a hollow appearance on Day 6 of the release study, with a polymer shell thickness of about 35% of the radius of the microspheres (Figure 1E). Furthermore, the polymer content in the 35% outer layer showed a linear correlation with the release constant from first-order model fitting of four in-house formulations (Figure 1F).
Conclusion: Microstructural properties (e.g., volume fraction and spatial distribution of polymer, drug, and pores) of microsphere formulations can be quantified using FIB-SEM combined with AI image analytics. Internal phase fractions and phase spatial distributions were identified that related to the in vitro release characteristics. Correlation between microstructural properties and in vitro release characteristics can potentially provide a more comprehensive understanding of the impact of microstructural properties on the underlying release mechanisms of microspheres, and aid in the establishment of Q3 equivalence.
References: [1]. Ruifeng, W., Quanying, B., Andrew G., et al. Int. J. of Pharm. 628, 122292.
Acknowledgements: Funding for this project was made possible, by the U.S. Food and Drug Administration through Contract # 75F40119C10157. The views expressed in this abstract do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services.
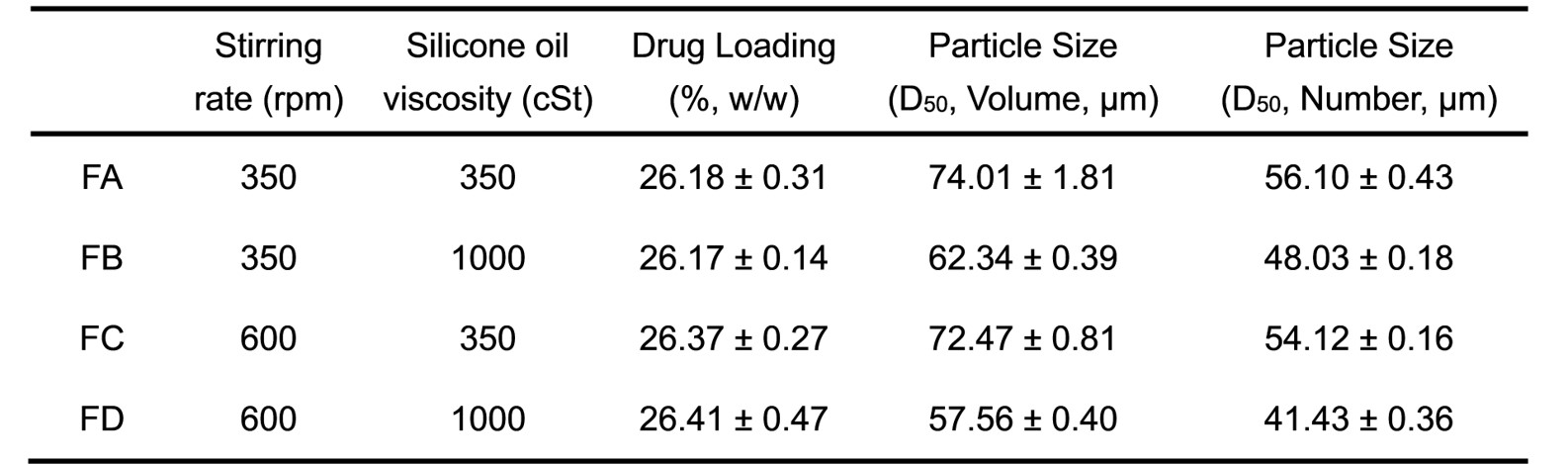 Table 1. Drug loading and particle size of the prepared microsphere formulations. All data are presented as mean ± SD (n=3).
Table 1. Drug loading and particle size of the prepared microsphere formulations. All data are presented as mean ± SD (n=3).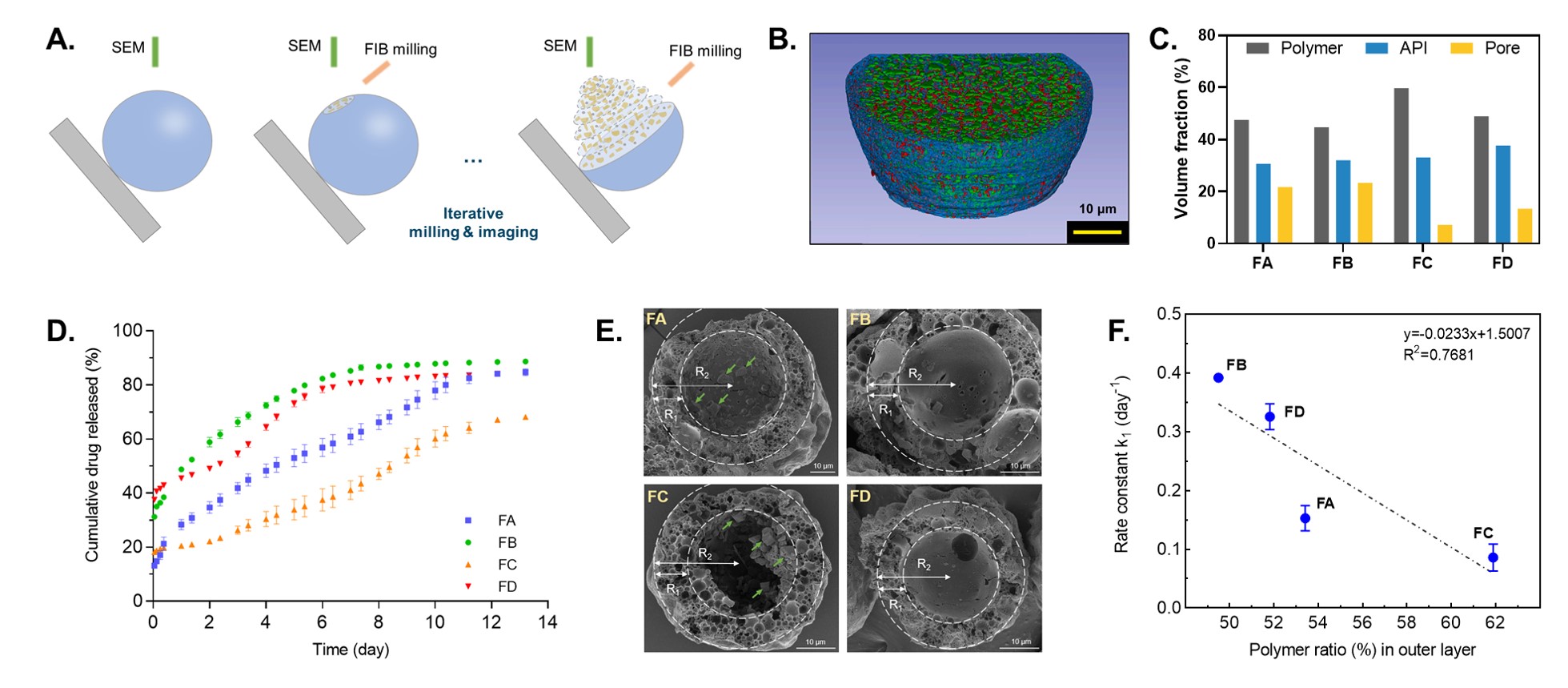 Figure 1. (A) Schematic overview of FIB-SEM imaging of a microsphere; (B) representative 3D reconstruction with polymer phase (blue), API phase (green), and pore phase (red); (C) 3D volume fractions of different phases of the prepared microsphere formulations; (D) in vitro release profiles of the prepared microspheres using the sample-and-separate method at 37℃ in 10 mM PBS (pH 7.4) containing 0.02% (v/v) Tween 20 (mean ± SD, n=3); (E) SEM cross-sectional images of the prepared microsphere formulations incubated in release medium at Day 6. The green arrows point to the remaining drug particles inside the microspheres. The ratio of R1/R2 is 35%; (F) correlation between the volume ratio of the polymer in the 35% outer shell layer and the release constant k1 using first-order model fitting for the prepared microsphere formulations.
Figure 1. (A) Schematic overview of FIB-SEM imaging of a microsphere; (B) representative 3D reconstruction with polymer phase (blue), API phase (green), and pore phase (red); (C) 3D volume fractions of different phases of the prepared microsphere formulations; (D) in vitro release profiles of the prepared microspheres using the sample-and-separate method at 37℃ in 10 mM PBS (pH 7.4) containing 0.02% (v/v) Tween 20 (mean ± SD, n=3); (E) SEM cross-sectional images of the prepared microsphere formulations incubated in release medium at Day 6. The green arrows point to the remaining drug particles inside the microspheres. The ratio of R1/R2 is 35%; (F) correlation between the volume ratio of the polymer in the 35% outer shell layer and the release constant k1 using first-order model fitting for the prepared microsphere formulations.