Formulation and Delivery - Biomolecular
Category: Poster Abstract
(M1030-01-05) Thrombocytopenia Protective Quetiapine Hemifumarate-Loaded Nasal Nanoemulgels for Schizophrenia
Monday, October 23, 2023
10:30 AM - 11:30 AM ET
- DG
Dnyandev G. Gadhave, Sr., Ph.D. (he/him/his)
St. John's University
New York, New York, United States - DG
Dnyandev G. Gadhave, Sr., Ph.D. (he/him/his)
St. John's University
New York, New York, United States - VG
Vivek Gupta, Ph.D. (he/him/his)
St. John's University
New York, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Schizophrenia is a complex mental condition that significantly impairs the patient's behavior, emotions, perception, and cognitive abilities. Over the past decade, the number of patients with schizophrenia has been increasing rapidly. Delivery of antipsychotic medicines to the brain is an extremely challenging task due to the hurdle of Blood-Brain barrier (BBB). Thus, conventional therapies and routes of administration used to treat many CNS disorders have multiple limitations and result in limited brain penetration. Quetiapine hemifumarate (QF), FDA-approved second-generation atypical antipsychotic, is potent against positive and negative symptoms of schizophrenia. However, contemporary reports have shown that long-term therapy with QF causes off-target effects such as lethal thrombocytopenia and leukopenia in schizophrenic patients. Hence, the present study aims to formulate QF-loaded chitosan-poloxamer in-situ gel (QF-Nanoemulgel) for localized brain delivery by nasal administration, and investigate its safety and therapeutic efficacy against schizophrenia.
Methods: QF-loaded nanoemulsion (QF-NE) was designed through a water titration technique. Box-Behnken statistical approach was employed to optimize the developed NE formulations. Developed formulations were followed by thermodynamic stability through a freeze-thaw cycle and centrifugation. Natural polymer chitosan (2%) and poloxamer 407 (18%) were utilized in combination to develop an in-situ gel (nanoemulgel) formulation. In this context, chitosan and poloxamer 407 aqueous solution was incorporated into the developed NEs at 4-8 ºCwith continuous stirring and placed to equilibrate for 10 min. Optimized formulations were then evaluated for globule size, polydispersity index, zeta potential, % transmittance (%T), viscosity, ex-vivo mucoadhesive strength, % entrapment efficiency (%EE), in-vitro cumulative QF release, and in-vitro hemolysis.
Results: According to the pseudo-ternary phase diagram a 3:1 ratio of surfactant: co-surfactant was found appropriate for the developed nanosystem, which revealed the highest nanoemulsion area (Fig. 1A). Experimental design was used to determine stable and suitable nanoformulations among all suggested 17 formulations and formulation F15 met all the criteria of nanoemulsion system, therefore formulation F15 was selected for further study (Figs. 1B-E). The thermodynamic stability studies reflected excellent stability after centrifugation and free-thaw stress conditions. Globule size, PDI, zeta potential, %T, viscosity, %EE, and ex-vivo mucoadhesive strength were found to be 15.0±0.32 nm, 0.054±0.0001, -18.03±0.15 mV, 99.8±0.8%, 13.5±2.1 cP, 69.0±1.5%, and 43.7±1.5 g (Figs. 2A-D), respectively. A cumulative in-vitro release study revealed 83.5±1.5% QF release through nanoemulgel after 8 h while 99.9±0.3% release occurred via QF-NE formulation and approximately 100% release occurred via both formulations after 24 h (Fig 2E). As can be seen, QF-nanoemulgel showed sustained release when compared to QF-NE and QF-suspension and obeyed zero-order kinetic (Fig. 2F). Nanoformulations (10, 50, and 100 μg/mL) treated blood samples did not cause any hemolysis as compared to drug and negative control, after 10 h treatment (Fig. 3). Further, in-vivo pharmacological studies are underway to justify in-vitro data.
Conclusion: Current research revealed the successful development of intranasal QF-Nanoemulgel as a novel dosage form for the safe and effective delivery of QF in schizophrenia patients.
.jpg) Figure 1: Optimization of nanoformulations: A) construction of pseudo-ternary phase diagram, B) risk analysis for the formulation of quetiapine hemifumarate nanoparticles, response surface plot for C) effects of formulation variables on globule size, D) effects of formulation variables on poly-dispersibility index and E) effects of formulation variables on zeta potential.
Figure 1: Optimization of nanoformulations: A) construction of pseudo-ternary phase diagram, B) risk analysis for the formulation of quetiapine hemifumarate nanoparticles, response surface plot for C) effects of formulation variables on globule size, D) effects of formulation variables on poly-dispersibility index and E) effects of formulation variables on zeta potential.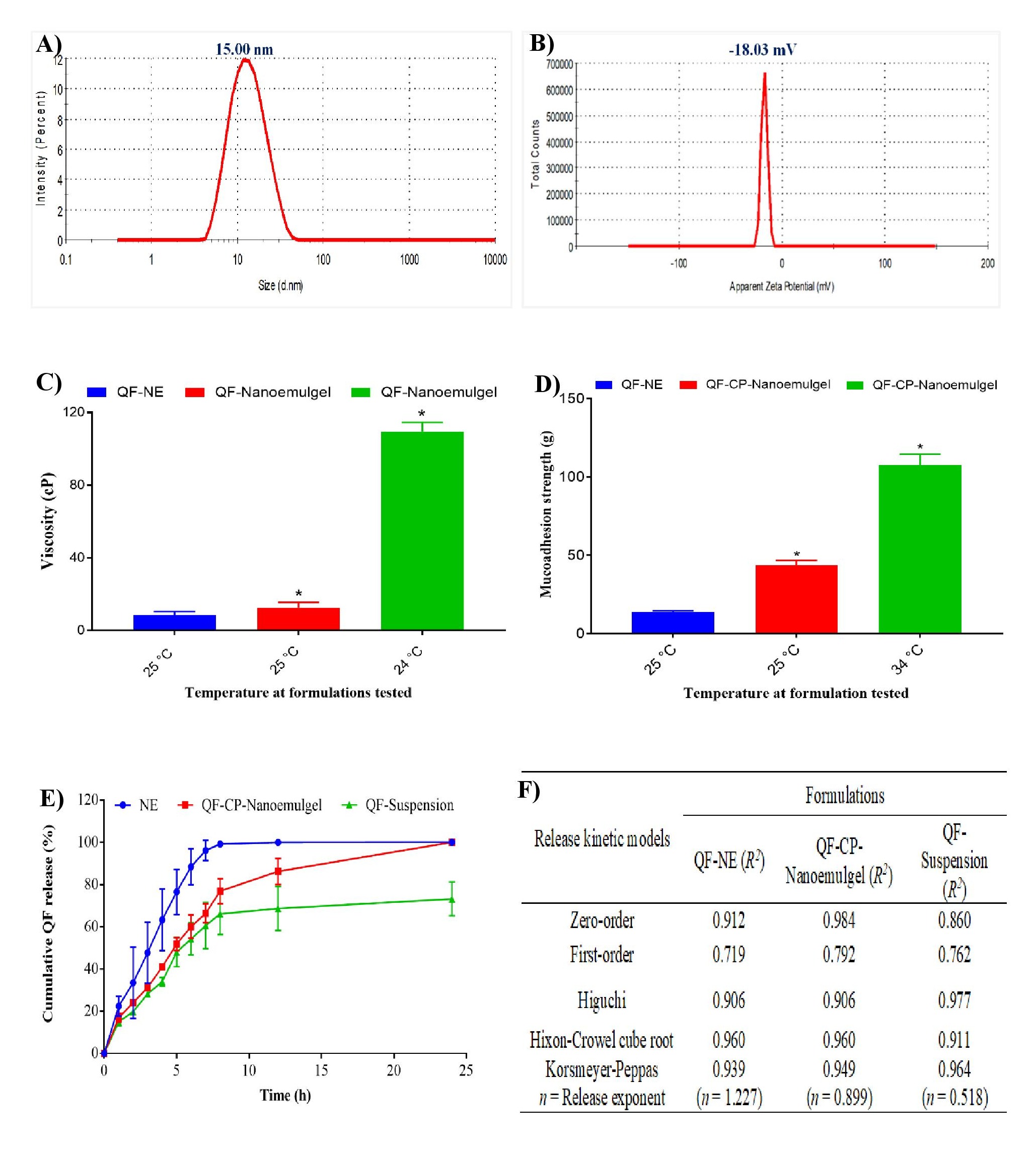 Figure 2: Evaluation of A) globule size, B) zeta potential, C) viscosity, D) ex vivo mucoadhesive strength, E) in vitro cumulative drug release study, and F) release kinetic study.
Figure 2: Evaluation of A) globule size, B) zeta potential, C) viscosity, D) ex vivo mucoadhesive strength, E) in vitro cumulative drug release study, and F) release kinetic study.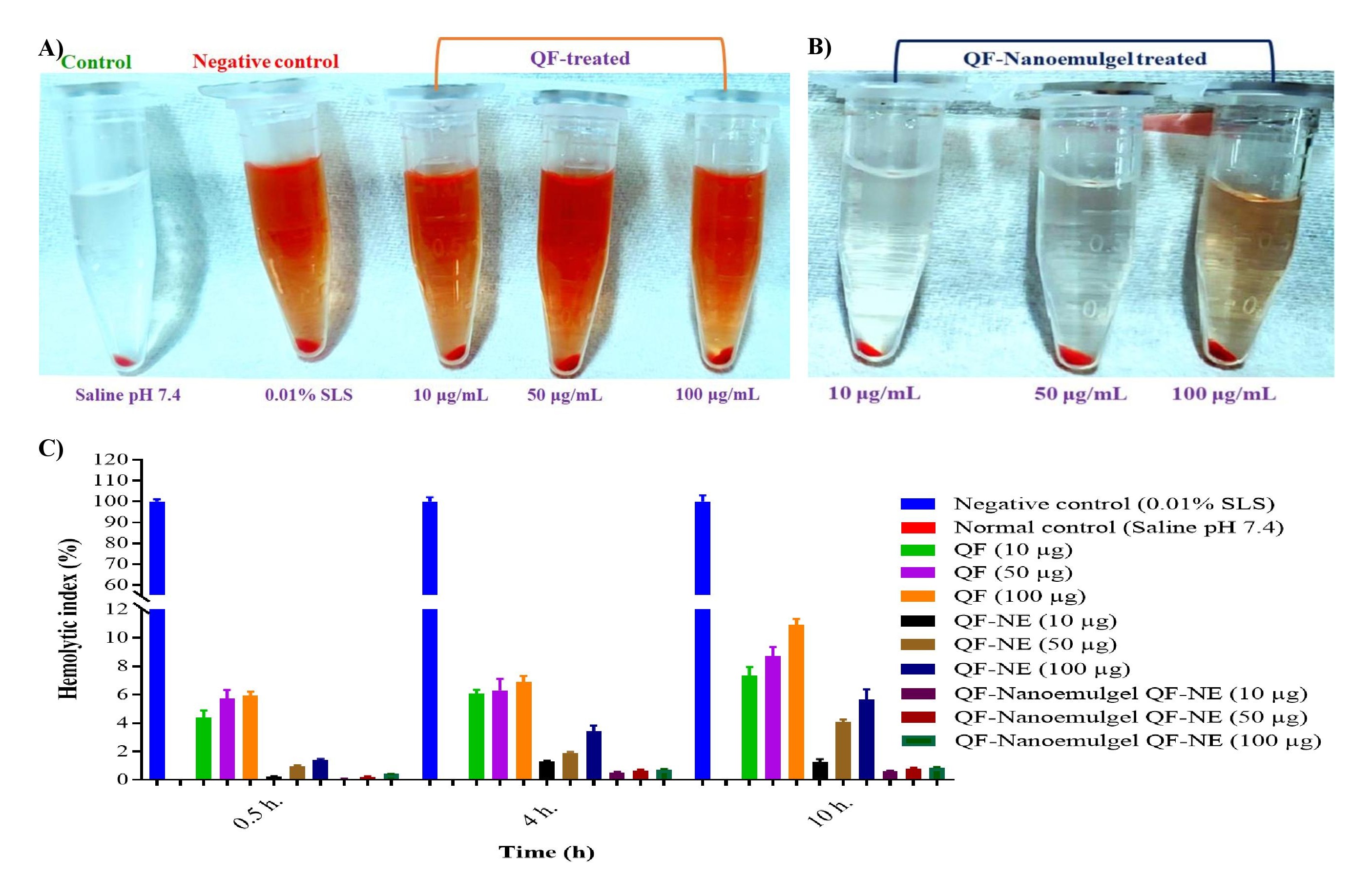 Figure 3: In vitro hemolysis study for developed QF-loaded nanoformulations: A) comparative hemolysis study of control (saline) against SLS-control (negative control) and pure QF at different concentrations (10, 50 and 100 μg/mL), B) hemolysis study for developed nanoformulation at different concentrations (10, 50 and 100 μg/mL), and C) % hemolysis index caused by different formulations.
Figure 3: In vitro hemolysis study for developed QF-loaded nanoformulations: A) comparative hemolysis study of control (saline) against SLS-control (negative control) and pure QF at different concentrations (10, 50 and 100 μg/mL), B) hemolysis study for developed nanoformulation at different concentrations (10, 50 and 100 μg/mL), and C) % hemolysis index caused by different formulations.