Formulation and Delivery - Chemical
Category: Poster Abstract
(M1430-02-11) Formulation and Evaluation of Prochlorperazine Maleate Sustained Release Mucoadhesive Buccal Films Using Hot Melt Extrusion
Monday, October 23, 2023
2:30 PM - 3:30 PM ET
- AK
Ajayreddy Kolipaka, MS
University of Mississippi
University, Mississippi, United States - UD
Umesh Chandra Devineni, MS
University of Mississippi
oxford, Mississippi, United States
Presenting Author(s)
Main Author(s)
Purpose: This study aims at developing a buccal film formulation containing the drug prochlorperazine maleate that can target the buccal mucosa. Prochlorperazine Maleate exhibits poor bioavailability when taken orally. Therefore, the buccal route was used in this study as this route gives us access directly into the systemic circulation, thereby surpassing the first-pass metabolism. The study intends to form the buccal film using hot melt extrusion, a process that involves mixing and melting the drug with a polymer matrix. The benefit of this approach is that this is a solvent-free and continuous process. The physical and mechanical properties and drug release kinetics of the buccal film formulations along with other analytical techniques were evaluated to determine the efficacy in delivering the drug in a sustained manner for the treatment of nausea and vomiting.
Methods: HME process was conducted using Thermo Scientific™ Minilab II 6mm Twin-Screw Extruder by Thermo Fisher Scientific (Waltham, MA, USA). The formulations were designed by keeping the drug load constant at 20% w/w as shown in the composition table. HPMC HME 4M was used as a sustained release agent, which was also found to have mucoadhesive properties. PVA and PEO were used in the formulation as film-forming polymers. Xanthan gum was used as a dedicated mucoadhesive agent and sorbitol was used as a plasticizer. A UV-visible spectrophotometer was used to analyze the formulations. In Vitro release was studied using USP Apparatus I, 900 ml of pH 6.8 Phosphate Buffer solution was used as the dissolution media as it closely mimics the pH of the buccal cavity and kept under continuous stirring at 37℃ ± 0.5℃ at 100 RPM. DSC and FTIR proved no unwanted interactions between drug and excipients.
Results: Prochlorperazine maleate was reported to have a maximum absorbance (λmax) at 255 nm. The torque in all processed formulations was between 20-30Nm which indicates that the formulations were malleable enough and suitable for HME processing. The DSC thermogram of the film obtained from formulation F4 suggests that there was no endothermic peak observed for Prochlorperazine maleate. FTIR analysis suggests that there were no interactions between the polymers and the drug. In Vitro, release testing results showed that the formulation F4 showed a 97% release rate at 2 hours time point. Formulation F3 which contains 20% HPMC showed less release (62%) when compared to F4. Formulation F5 and F6 exhibited over 90% release but, a burst release was observed after 15 minutes till 30 minutes. Formulations F7, F8, F9, and F10 also showed burst release at the initial time point of 15 minutes. This burst release can be attributed to the low or no HPMC content. The dissolution profiles of formulations F3 to F10 were depicted in the figure.
Conclusion: Prochlorperazine Maleate mucoadhesive buccal films were successfully prepared and evaluated. The films obtained from formulation F4 with 15% HPMC showed the desired release rate at the end of 2 hours and this was considered as the optimal formulation. Evaluation test results were found to be satisfactory and there were no significant observations or changes noticed. This study demonstrates that the Hot Melt Extrusion technique has excellent potential for the preparation of mucoadhesive buccal films. These HME films can significantly reduce the amount of drug intake and the frequency of the dosage, thereby enhancing patient compliance and the therapeutic efficacy of the drug.
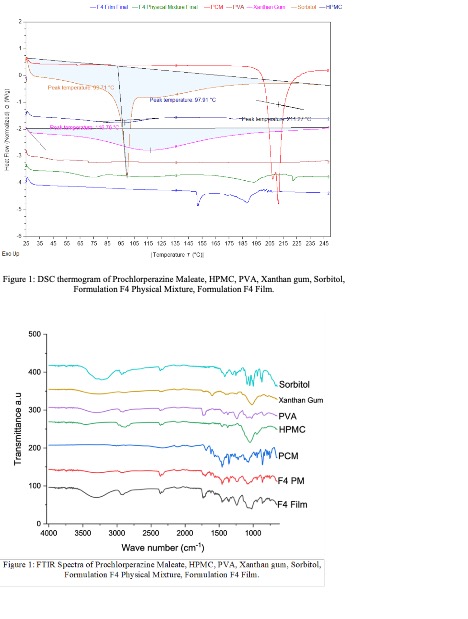 DSC and FTIR studies
DSC and FTIR studies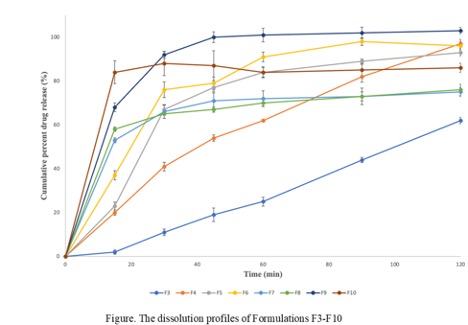 The dissolution profiles
The dissolution profiles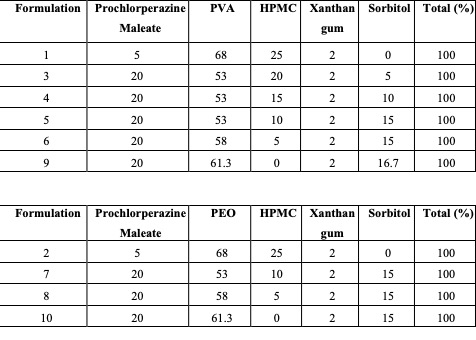 Composition table
Composition table