Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(W1230-03-18) Stimuli-Responsive Newly Designed Poly (amino ether) Glycopolymer Based Pegylated Nanoparticles Conjugated with Receptor Binding Peptide for siRNA Delivery to Treat Lung Cancer
Wednesday, October 25, 2023
12:30 PM - 1:30 PM ET
- AK
Arun Kumar Kotha, Ph.D. (he/him/his)
Mercer University
Atlanta, Georgia, United States - NG
Nishant Gandhi, M.S. (he/him/his)
University of Hawaii at Hilo
Hilo, Hawaii, United States - SG
Sudhakar Godeshala, Ph.D. (he/him/his)
Arizona State University
Tempe, Arizona, United States - DK
Dana-Lynn Koomoa-Lange, Ph.D. (she/her/hers)
University of Hawaii at Hilo
Hilo, Hawaii, United States - BM
Bhavani Miryala, Ph.D. (she/her/hers)
Arizona State University
TEMPE, Arizona, United States - KR
Kaushal Rege, Ph.D. (he/him/his)
Arizona State University
Tempe, Arizona, United States - MC
Mahavir Chougule, Ph.D. (he/him/his)
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The mTOR siRNA (MS) has been shown to inhibit Non-small cell lung cancer (NSCLC) cells, in addition to inducing apoptosis. But its use is limited due to susceptibility to degradation by nucleases, poor cellular uptake, and delivery challenges. Lung cancer is the leading cause of cancer related fatalities around the world. NSCLC is the most common form of lung cancer. General treatment methods, i.e., surgery, chemotherapy, radiation, and immunotherapy, does not provide safe and efficacious treatment. To overcome the challenges, stimuli-responsive and pH-sensitive MS loaded modified poly (amino ether) (MP) glycopolymeric based pegylated (PG) nanoparticles (NPs) were developed, which have endosomal escape capability and glutathione (GSH) cleavable disulfide linkage for triggered MS release. NPs conjugated with Luteinizing Hormone-Releasing Hormone Receptor (LHRH-R) binding peptide (LR), will selectively enhance the cellular uptake in LHRH-R overexpressing NSCLC cells and exert a significant anticancer effect. The objective was to design and synthesize of GSH responsive poly (amino ether) pegylated glycopolymer and use it to formulate, characterize, and evaluate the MS-loaded MP-PG NPs conjugated with LR for the targeted delivery to down regulate mTOR gene and inhibit the growth of lung cancer.
Methods: Modified PAE (MP) polymer with pH sensitive and GSH stimuli-responsive property was synthesized by a simple one-step reaction with Traut’s reagent as described previously (1). The MS encapsulated MP-PG-LR NPs were prepared by a modified nanoprecipitation method Fig 1A. Initially MS-MP NPs suspension was prepared, and they were surface modified with PEG and LR (MS-MP-PG-LR) by a two-step reaction (Figure 1A). Particle size, PDI, and zeta potential (ZP) of MS-MP-PG and MS-MP-PG-LR-20 NPs were analyzed using NICOMP 380ZLS. In vitro cytotoxicity of NPs was conducted in A549 and H460 cells using a modified SRB assay. Intracellular trafficking of fluorescein isothiocyanate (FITC) conjugated siRNA (FS)-MP-PG-LR NPs was evaluated in A549 cells at various time points (0.5 h, 2 h, and 6h) by 40X confocal imaging. Evaluation of Cellular uptake of FS-MP-PG-LR NPs at different LR concentrations was analyzed in A549, H460, minimal LHRH-R expressing SKOV-3 cell lines by BD ACCURI C6 PLUS flow cytometer. In vitro mTOR gene silencing efficiency of NPs was analyzed in A549 and H460 cells by SDS-PAGE and Western blotting.
Results: MS-MP-PG NPs size was 124±5.9nm with a PDI of 0.37, zeta potential of 19.5±4.6mV. After LR conjugation (MS-MP-PG-LR), NPs size increased slightly to 132±6.8nm with PDI of 0.32 and zeta potential of 20.2±5.4nm. Non-targeted MP-NP formulations showed cytotoxicity at 60µg/ml in both A549 and H460 cells (65.4±10.4%, 60.81± 8.59%, respectively). PEGylated non-targeted and targeted formulations did not show cytotoxicity at all analyzed concentrations, which indicates that PEGylation helped in decreasing cytotoxicity of NPs (Figure 1B). Intracellular localization of the NPs was evaluated by tracking the bright green fluorescence of FS. The nuclei and the acidic late endosomes/early lysosomes were stained with Hoescht 33342 and LysoTracker Red, respectively. From figure 2A, it can be observed that maximum levels of co-localization could be observed after 2 h when almost all of the FS was located in the endosome/lysosome. By the end of 6 h, some of the bio-reducible NPs escaped the endosome/lysosomes to reach the cytoplasm and release the siRNA due to the presence of cytoplasmic GSH. The amount of siRNA colocalized in the endo/lysosomes was about 5.75 ± 1.2%, 45.7 ± 8.5%, and 59.3 ± 12.5 % at times 0.5 h, 2 h, and 6 h, respectively. As shown in Figure. 2B (A and B), the cellular uptake efficiency of FS-MP-PG-LR20 NPs was significantly higher than the non-targeted FS-MP-PG NPs in A549 cells(p < 0.001) and also in H460 cells (p < 0.001) (C and D). There was no significant difference between the cellular uptake in FS-MP-PG-LR20 NPs and FS-MP-PG-LR40 NPs. It was observed (E and F) that there was no significant difference in cellular uptake of FS-MP-PG NPs and FS-MP-PG-LR20 NPs in the minimal LHRH-R expressing SKOV-3 cell lines (p >0.05), which confirms that the LHRH receptor is an essential property for the effective internalization of the targeted NPs. After treatment with MS-MP-PG-LR20 NPs in both A549 and H460 cells (normalized to the β-actin expression) at 50 nM and 100 nM concentrations, the expression of the mTOR protein was significantly decreased (~67% and 70% mTOR knockdown for A549 and ~70% and 75% mTOR knockdown for H460 cells) than SS-MP-PG-LR20 NPs (**p < 0.01) (Figure. 3 (A, B) and (C, D)).
Conclusion: MP polymer produced nano-sized and cationic NPs loaded with MS. The PEGylation of NPs (PEG, 5K) resulted in decreased cytotoxicity in NSCLC cells. NPs showed endosomal escape and cytoplasmic GSH triggered siRNA release. LR helps in enhancing selective cellular uptake via receptor-mediated endocytosis. MS-MP-PG-LR efficiently silenced the mTOR gene in A549 and H460 cells. The novel receptor binding peptide conjugated polymer mPAE for mTOR siRNA delivery NPs showed potential for treating NSCLC.
References: Gandhi NS, Godeshala S, Koomoa-Lange DT, Miryala B, Rege K, Chougule MB. Bioreducible Poly(Amino Ethers) Based mTOR siRNA Delivery for Lung Cancer. Pharm Res. 2018 Aug 13;35(10):188. doi: 10.1007/s11095-018-2460-z. Erratum in: Pharm Res. 2018 Sep 5;35(11):202. PMID: 30105526; PMCID: PMC8463011.
Acknowledgements: Research findings reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number SC3GM109873 to Dr. Mahavir Chougule’s lab. Dr. Chougule’s research team would like to acknowledge the partial support by National Heart, Lung, and Blood Institute of the National Institutes of Health award, R15HL138718. Also, Dr. Mahavir Chougule’s lab would like to acknowledge startup funds support from Mercer University College of Pharmacy. Dr. Gandhi acknowledges the Daniel K. Inouye College of Pharmacy, University of Hawaii at Hilo, Hilo, HI 96720, USA, for providing graduate assistantship. Dr. Rege’s group is grateful to the NIH/NCATS (Grant 1R41TR003247-01) and the Arizona Biomedical Research Centre (ABRC; Grant number CTR056043), which partially facilitated this study. Declaration of Interest: Dr. Rege is affiliated with Synergyan, LLC
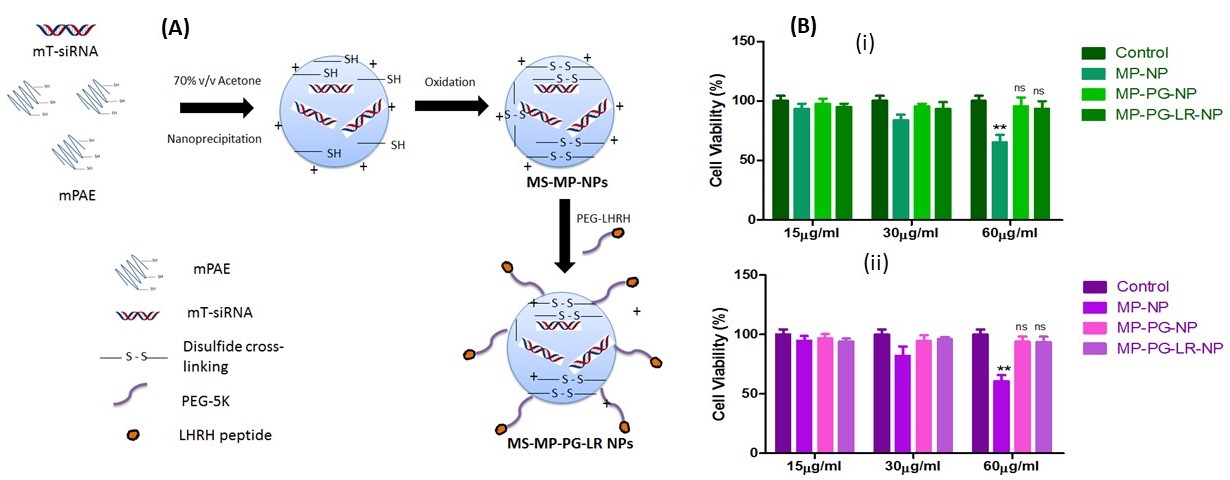 Figure 1A: Scheme for the formation of disulfide cross-linked mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR NPs)
Figure 1A: Scheme for the formation of disulfide cross-linked mTOR siRNA-mPAE-PEG-LHRH-R binding peptide nanoparticles (MS-MP-PG-LR NPs)Figure 1B: In vitro cell viability of MP-NP, MP-PG-NP and MP-PG-LR-NP in
(i) A549 and (ii) H460 cells at various concentrations (15µg/ml, 30 µg/ml, and 60 µg/ml) following incubation period of 48h.
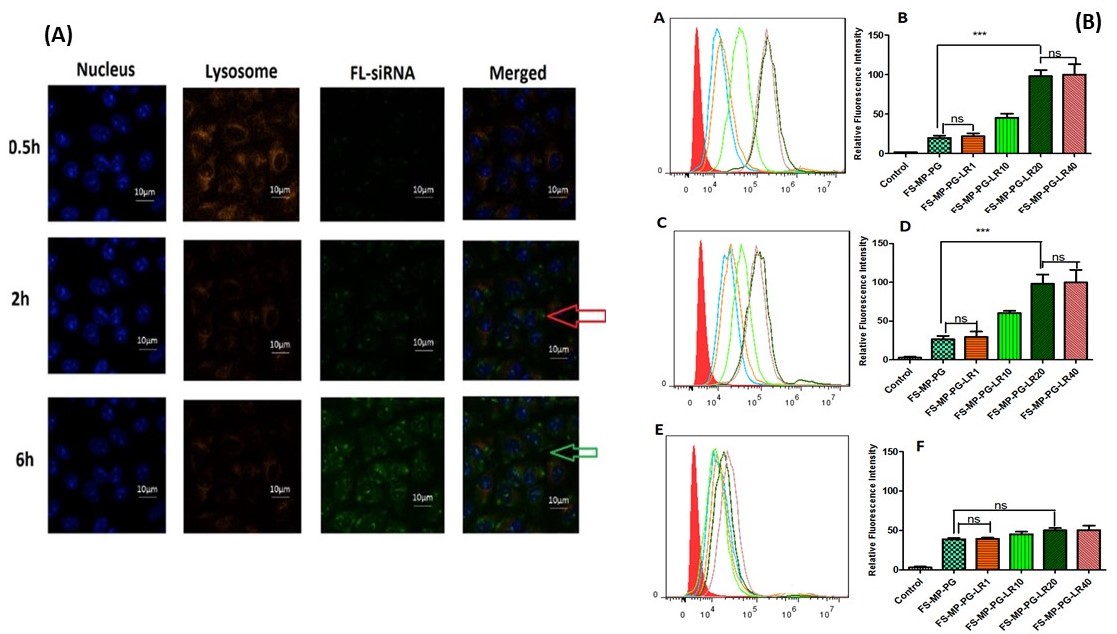 Figure 2A: Intracellular localization of FS-MP-PG-LR20 NPs (60µg/ml) in A549 cells (Red arrow indicates the colocalization of FS-MP-PG-LR20 and LysoTracker Red; Green arrow indicates the presence of FS in the cytosol).
Figure 2A: Intracellular localization of FS-MP-PG-LR20 NPs (60µg/ml) in A549 cells (Red arrow indicates the colocalization of FS-MP-PG-LR20 and LysoTracker Red; Green arrow indicates the presence of FS in the cytosol).Figure 2B: Cell binding and uptake analysis NPs by FACS. The fluorescence intensity of FS-MP-PG-NPs and FS-MP-PG-LR-NPs (1, 10, 20 and 40µM) in (A and B) A549, (C and D) H460, (E and F) SKOV-3 cells (5 × 103 cell/well). Bar graph representing the mean percentages of cellular uptake. The results are presented as mean ± SD (n=3) (*** p < 0.001; ** p < 0.01; *p < 0.05; ns = Non- significant, p >0.05).
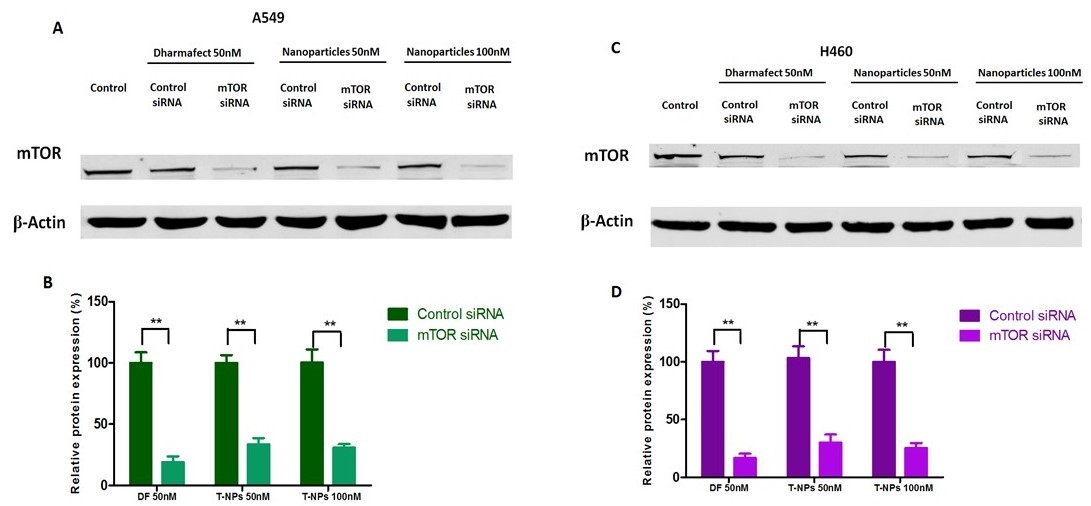 Figure 3: Silencing efficiency of MS-DF transfection reagent and MS-MP-PG-LR20 NPs at 50nM and 100nM (siRNA) concentration. Western blot showed a marked reduction in the mTOR protein levels after treatment with MS-MP-PG-LR20 NPs, whereas the negative control (SS-MP-PG-LR20 NPs) did not show gene silencing effects in A549 and H460 cells (A) and (C). mTOR protein silencing was calculated using the densitometric analysis and β-Actin as the loading control (B) and (D). (SS: scrambled siRNA; T-NP: Targeted nanoparticles). The results are presented as mean ± SD (n=3) (** p < 0.01).
Figure 3: Silencing efficiency of MS-DF transfection reagent and MS-MP-PG-LR20 NPs at 50nM and 100nM (siRNA) concentration. Western blot showed a marked reduction in the mTOR protein levels after treatment with MS-MP-PG-LR20 NPs, whereas the negative control (SS-MP-PG-LR20 NPs) did not show gene silencing effects in A549 and H460 cells (A) and (C). mTOR protein silencing was calculated using the densitometric analysis and β-Actin as the loading control (B) and (D). (SS: scrambled siRNA; T-NP: Targeted nanoparticles). The results are presented as mean ± SD (n=3) (** p < 0.01).