Preclinical and Translational Sciences - Biomolecular
Category: Late Breaking Poster Abstract
(W1130-10-66) Multiscale Modeling-Identified Synergistic Combinations of Anti-microRNA-155 and Standard-of-Care Drugs for Improved Outcomes in Non-small Cell Lung Cancer
Wednesday, October 25, 2023
11:30 AM - 12:30 PM ET

Prashant Dogra, PhD (he/him/his)
Assistant Research Professor
Houston Methodist Research Institute
Houston, Texas, United States
Prashant Dogra, PhD (he/him/his)
Assistant Research Professor
Houston Methodist Research Institute
Houston, Texas, United States- JC
Joseph Cave, M.S.
Houston Methodist Research Institute
Houston, Texas, United States - JB
Joseph Butner, Ph.D.
Houston Methodist Research Institute
Houston, Texas, United States - VC
Vittorio Cristini, Ph.D.
Houston Methodist Research Institute
Houston, Texas, United States - ZW
Zhihui Wang, Ph.D.
Houston Methodist Research Institute
Houston, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Non-small cell lung cancer (NSCLC), which accounts for 80% of lung cancer cases, is the leading cause of cancer-related deaths in the United States. Development of resistance is the major cause of failure of the platinum-based standard-of-care (SOC) chemotherapies, leading to relapses and patient deaths. It has been shown that microRNA-155 (miR-155) overexpression is associated with chemotherapy resistance, aggressiveness, and poor prognosis in NSCLC [1, 2]; miRNAs are small non-coding RNAs that regulate gene expression by binding to messenger RNAs (mRNAs) and inhibiting their translation. Dysregulation of miR expression has been implicated in cancer development due to its upregulation or downregulation of oncogenic [3] or tumor suppresser effects [4], respectively. It is known that miR-155 induces chemoresistance through a TP53 feedback loop, and that in vivo therapeutic targeting of miR-155 (through its antagonist, anti-miR-155) significantly enhances the efficacy of chemotherapy, thereby providing rational for this therapeutic approach [5]. However, miR-155 also suppresses PD-L1 expression in NSCLC [6], so we anticipate a negative effect of anti-miR-155 therapy on tumor immunosurveillance. To help maximize the therapeutics benefits of anti-miR-155 while minimizing the adverse reactions, we have developed a mathematical model to facilitate the optimal dosing and combinations of anti-miR-155 with SOC drugs in NSCLC.
Methods: We developed a multiscale mechanistic model of tumor growth dynamics to simulate the pharmacokinetics (PK) and pharmacodynamics (PD) of nanoparticle (NP)-delivered anti-miR-155 therapy in NSCLC based on our previous work [7-9], and evaluated its translational potential to improve treatment outcomes alone and when used in combination with SOC chemotherapy and/or immune checkpoint inhibitor (ICI) immunotherapy. The current model consists of two major compartments representing the plasma and tumor, where the latter comprises cancer cells and infiltrating immune cells, specifically tumor-associated macrophages (TAMs) and CD8+ T-cells (Figure 1A). The model is formulated as a system of ordinary differential equations that characterize the relevant transport phenomena associated with nanoparticle-mediated delivery of anti-miR-155 into cancer cells and TAMs, cellular-scale tumor-immune interactions, and the molecular-scale effects of anti-miR-155 on cancer dynamics.
Results: The model was solved numerically in MATLAB and calibrated with published in vivo datasets on NSCLC-bearing mice treated with anti-miRNA-155 and other SOC drugs (cisplatin, atezolizumab, and pembrolizumab; Figure 1B-H). In Figure 1B, we observe that before anti-miR-155 treatment, the model-predicted concentration of miR-155 saturates to just below 1.5 pM in cancer cells and TAM sub-compartments. Following twice weekly injection of a 4000 ng dose of anti-miR-155 (encapsulated in NPs), the concentration of miR-155 in cancer cells and TAM sub-compartments drops, as expected from the antagonistic effect of anti-miR-155. Furthermore, we observed that the concentration of PD-L1 increases by an order of magnitude, as miR-155 is a negative regulator of PD-L1 expression; however, the concentration of PD-1 in T-cells remains constant at 1 pM, as the regulation of PD-1 expression is not explicitly affected by miR-155 (Figure 1C). The mass of NPs in the cancer cell and TAM sub-compartment volumes initially rises with each dose before gradually retreating (Figure 1D). We also observed a similar trend in the concentration of anti-miR-155 in the tumor cells and TAM (Figure 1E). Finally, model fits of volumetric tumor growth show excellent agreement with experimental data, with Pearson correlation coefficient R > 0.99 (Figure 1F-H). It is evident that both atezolizumab and pembrolizumab treatments act to reduce the tumor volumes (Figure G,H), and in the case of anti-mir-155 and cisplatin, we observe that combination therapy has a more significant effect than monotherapy (Figure F). Following model calibration to in vivo murine data, allometric scaling of the model to humans was performed and virtual cohorts of stage IV NSCLC-bearing patients were generated using the Allen method [10]; model predictions of progression free survival (PFS) based on RECIST 1.1 [11] were validated with results from clinical trials using a SOC drug (atezolizumab) [12] with good agreement between measured and model predicted outcomes (Figure 2A). Finally, drug synergism was evaluated for novel combinations of anti-miR-15 with SOC drugs using the Chou-Talalay method [13] in virtual patient cohorts to identify novel drug combinations that may improve clinical outcomes with reduced dose and frequency of usage of SOC drugs in NSCLC. As observed in Figure 2B,C, we identified two potent drug combinations of anti-miR-155, one with atezolizumab (Figure 2B) and the other with cisplatin and pembrolizumab (Figure 2C) that reduce the clinically required dose and frequency and lead to improved PFS outcomes than their corresponding monotherapy scenarios.
Conclusion: This study presents a novel mathematical model for investigating the translational potential of anti-miR-155 nanotherapy for NSCLC in combination with SOC drugs. Our computational analysis revealed synergistic combination strategies for improving treatment outcomes, providing a basis for identifying rational therapeutic combinations. As a result, our approach may serve as a useful computational means to help design and optimize a therapeutic framework for future clinical trials of anti-miR-155 and other gene therapies.
References: 1. Xue X, Liu Y, Wang Y, Meng M, Wang K, Zang X, et al. MiR-21 and MiR-155 promote non-small cell lung cancer progression by downregulating SOCS1, SOCS6, and PTEN. Oncotarget. 2016;7(51):84508-19. Epub 2016/11/05. doi: 10.18632/oncotarget.13022. PubMed PMID: 27811366; PubMed Central PMCID: PMCPMC5356677.
2. Zang YS, Zhong YF, Fang Z, Li B, An J. MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin via negative regulation of Apaf-1 expression. Cancer Gene Therapy. 2012;19(11):773-8. doi: 10.1038/cgt.2012.60.
3. Frixa T, Donzelli S, Blandino G. Oncogenic MicroRNAs: Key Players in Malignant Transformation. Cancers (Basel). 2015;7(4):2466-85. Epub 20151218. doi: 10.3390/cancers7040904. PubMed PMID: 26694467; PubMed Central PMCID: PMCPMC4695904.
4. Otmani K, Lewalle P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front Oncol. 2021;11:708765. Epub 20211015. doi: 10.3389/fonc.2021.708765. PubMed PMID: 34722255; PubMed Central PMCID: PMCPMC8554338.
5. Van Roosbroeck K, Fanini F, Setoyama T, Ivan C, Rodriguez-Aguayo C, Fuentes-Mattei E, et al. Combining Anti-Mir-155 with Chemotherapy for the Treatment of Lung Cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(11):2891-904. Epub 2016/12/03. doi: 10.1158/1078-0432.Ccr-16-1025. PubMed PMID: 27903673; PubMed Central PMCID: PMCPMC5449263.
6. Huang J, Weng Q, Shi Y, Mao W, Zhao Z, Wu R, et al. MicroRNA-155-5p suppresses PD-L1 expression in lung adenocarcinoma. FEBS Open Bio. 2020;10(6):1065-71. Epub 2020/04/03. doi: 10.1002/2211-5463.12853. PubMed PMID: 32237066; PubMed Central PMCID: PMCPMC7262882.
7. Dogra P, Ramírez JR, Butner JD, Peláez MJ, Chung C, Hooda-Nehra A, et al. Translational Modeling Identifies Synergy between Nanoparticle-Delivered miRNA-22 and Standard-of-Care Drugs in Triple-Negative Breast Cancer. Pharmaceutical Research. 2022. doi: 10.1007/s11095-022-03176-3.
8. Dogra P, Ramírez JR, Butner JD, Peláez MJ, Cristini V, Wang Z, editors. A Multiscale Model to Identify Limiting Factors in Nanoparticle-Based miRNA Delivery for Tumor Inhibition. 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); 2021 1-5 Nov. 2021.
9. Cave J, Shinglot V, Butner JD, Vittorio C, Ozpolat B, Calin GA, et al. Mechanistic modeling of anti-microRNA-155 therapy combinations in lung cancer. 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); 24-27 July, 2023; Sydney, Australia 2023.
10. Allen RJ, Rieger TR, Musante CJ. Efficient Generation and Selection of Virtual Populations in Quantitative Systems Pharmacology Models. CPT Pharmacometrics Syst Pharmacol. 2016;5(3):140-6. Epub 2016/04/14. doi: 10.1002/psp4.12063. PubMed PMID: 27069777; PubMed Central PMCID: PMCPMC4809626.
11. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer. 2009;45(2):228-47.
12. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. New England Journal of Medicine. 2020;383(14):1328-39. doi: 10.1056/NEJMoa1917346. PubMed PMID: 32997907.
13. Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27-55.
Acknowledgements:The research work was supported in part by the Cockrell Foundation (V.C., P.D.), the National Institutes of Health (NIH) Grants 1R03EB033576 (P.D.), 1R01CA253865 (V.C., Z.W.), 1R01CA222007 (V.C., Z.W.), and 1R01CA226537 (V.C., Z.W.).
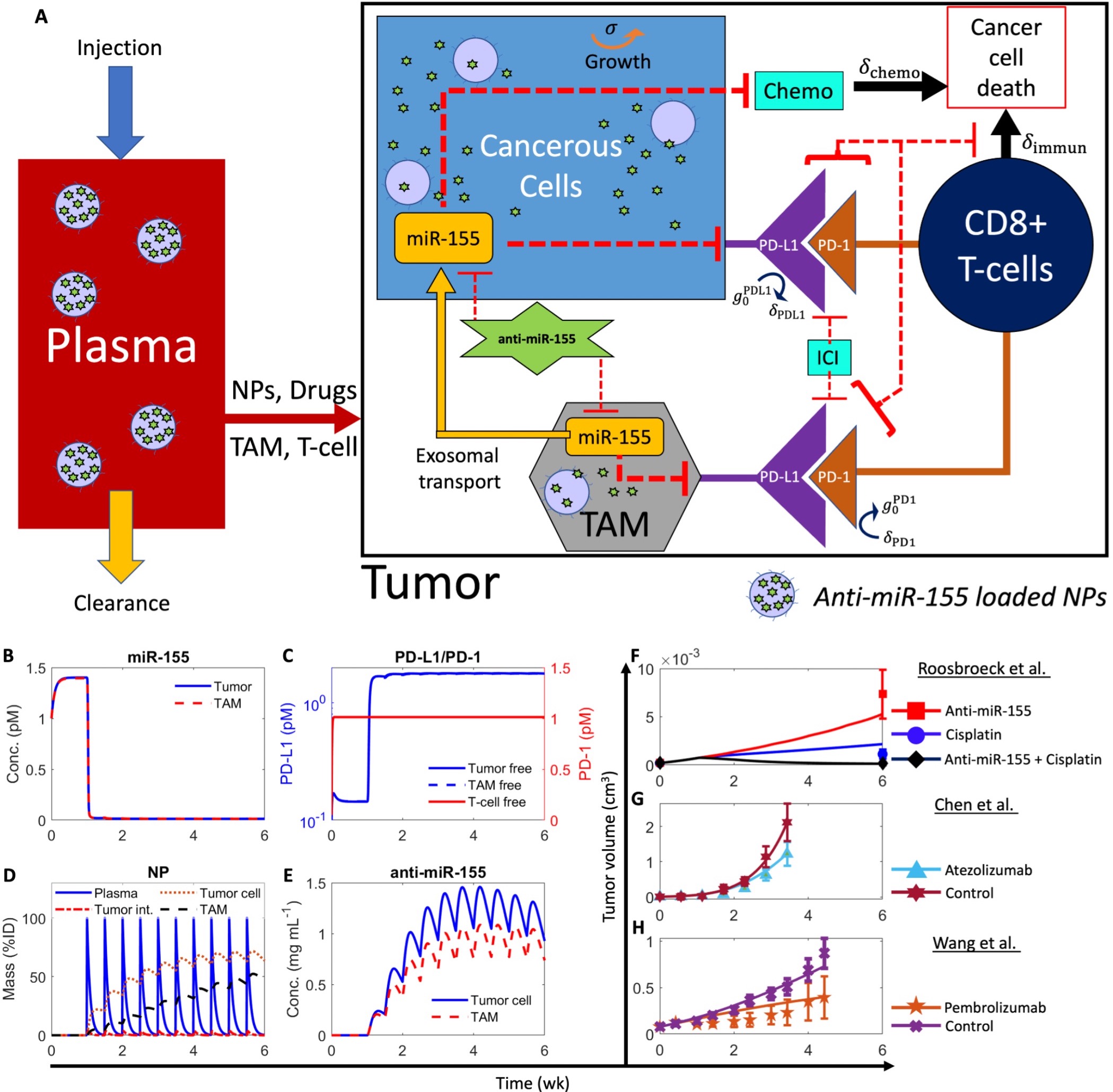 Figure 1. Model schematic and calibration. A) Model schematic showing key variables and system interactions. B-E) Numerical solution of the model exhibiting kinetics of key variables under treatment with NP-delivered anti-miR-155. B) Concentration kinetics of miR-155 in cancer cells and TAMs. C) Concentration kinetics of unbound (i.e., free) PD-L1 on tumor cells and TAM (left y-axis), and unbound PD-1 on CD8+ T-cells (right y-axis). D) Mass kinetics of NPs in plasma, tumor interstitium, tumor cells, and TAMs following twice weekly injection of NPs loaded with a dose of 4000 ng of anti-miR-155. %ID represents percent of injected dose. E) Concentration kinetics of the NP-delivered anti-miR-155 in tumor cells and TAMs. F-H) Model fits to published in vivo datasets of tumor volumetric growth kinetics of NSCLC under control conditions or treatment with F) anti-miR-155, cisplatin, combination of anti-miR-155 and cisplatin, G) atezolizumab, and H) pembrolizumab.
Figure 1. Model schematic and calibration. A) Model schematic showing key variables and system interactions. B-E) Numerical solution of the model exhibiting kinetics of key variables under treatment with NP-delivered anti-miR-155. B) Concentration kinetics of miR-155 in cancer cells and TAMs. C) Concentration kinetics of unbound (i.e., free) PD-L1 on tumor cells and TAM (left y-axis), and unbound PD-1 on CD8+ T-cells (right y-axis). D) Mass kinetics of NPs in plasma, tumor interstitium, tumor cells, and TAMs following twice weekly injection of NPs loaded with a dose of 4000 ng of anti-miR-155. %ID represents percent of injected dose. E) Concentration kinetics of the NP-delivered anti-miR-155 in tumor cells and TAMs. F-H) Model fits to published in vivo datasets of tumor volumetric growth kinetics of NSCLC under control conditions or treatment with F) anti-miR-155, cisplatin, combination of anti-miR-155 and cisplatin, G) atezolizumab, and H) pembrolizumab. 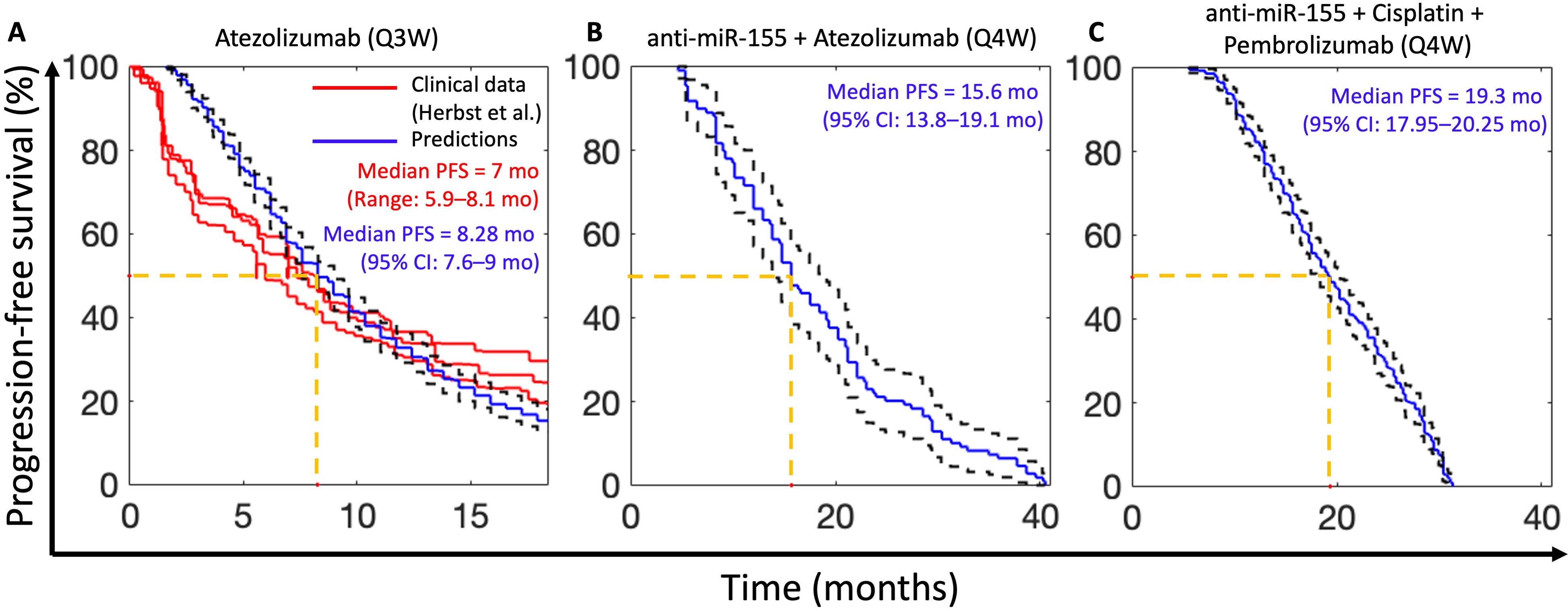 Figure 2. Clinical model validation and predictions of PFS for novel drug combinations with synergistic effects. A) Model predicted (blue) PFS for treatment of 1,000 virtual patients with 1,200 mg Q3W atezolizumab in comparison with published clinical trial data (red) for the same dosage of the drug. B) PFS predictions for combination of anti-miR-155 + atezolizumab (Q4W) given concomitantly for 46 cycles at doses of 0.828 mg/kg and 16.98 mg/kg, respectively. C) PFS predictions for combination of anti-miR-155 (35 cycles) + cisplatin (5 cycles) + pembrolizumab (35 cycles) at Q4W frequency given concomitantly at doses of 0.458 mg/kg, 1.827 mg/kg, and 2.181 mg/kg, respectively. Dashed black curves represent 95% CI. Note that the number of cycles chosen were based on published clinical studies for standard-of-care drugs. Abbreviation: Q3W- once in three weeks, Q4W- once in four weeks.
Figure 2. Clinical model validation and predictions of PFS for novel drug combinations with synergistic effects. A) Model predicted (blue) PFS for treatment of 1,000 virtual patients with 1,200 mg Q3W atezolizumab in comparison with published clinical trial data (red) for the same dosage of the drug. B) PFS predictions for combination of anti-miR-155 + atezolizumab (Q4W) given concomitantly for 46 cycles at doses of 0.828 mg/kg and 16.98 mg/kg, respectively. C) PFS predictions for combination of anti-miR-155 (35 cycles) + cisplatin (5 cycles) + pembrolizumab (35 cycles) at Q4W frequency given concomitantly at doses of 0.458 mg/kg, 1.827 mg/kg, and 2.181 mg/kg, respectively. Dashed black curves represent 95% CI. Note that the number of cycles chosen were based on published clinical studies for standard-of-care drugs. Abbreviation: Q3W- once in three weeks, Q4W- once in four weeks.