Manufacturing and Analytical Characterization - Biomolecular
Category: Late Breaking Poster Abstract
(W0930-09-56) Potency Assay Development for Gene Therapy Products – Approaches and Points to Consider in Data Processing
Wednesday, October 25, 2023
9:30 AM - 10:30 AM ET
- JP
Jeff Patrick, PhD
Senior Director of GMP Operations
BioAgilytix - Durham, NC
Durham, North Carolina, United States - JP
Jeff Patrick, PhD
Senior Director of GMP Operations
BioAgilytix - Durham, NC
Durham, North Carolina, United States - LP
Lakshmi Pillai-Kastoori, Ph.D.
BioAgilytix
Durham, North Carolina, United States - GJ
Grant Jones, Ph.D.
BioAgilytix
Durham, North Carolina, United States - RD
Reema Davis, Ph.D.
BioAgilytix
Durham, North Carolina, United States - JW
Jessica Weaver, M.S.
BioAgilytix
Durham, North Carolina, United States - BJ
Bhoomi Jani, Ph.D.
BioAgilytix
Durham, North Carolina, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Co-Author(s)
Purpose: Gene therapies – including AAVs, engineered viruses and polymer formulated oligonucleotides/plasmids – is among the highest growth area in biopharmaceuticals. They provide great promise as therapeutics but present many challenges owing to their complex mechanisms of action (MOA). Potency assays play a pivotal role in the development of nearly every biologic and are an expectation of global regulatory agencies. These assays take many forms depending on the MOA of the biologic and include analysis of cell activity using ELISAs, Flow Cytometry, and ddPCR/qPCR. The cells and the readout are critical but so is the appropriate processing of the data to derive a relative potency. Here case studies for representative gene therapies have been provided as examples of the challenges encountered in acquiring and processing data. Different data modelling is applied to achieve robust outcomes that meet the requirements of a GMP Relative Potency assay. We include discussion of phase appropriateness and the risks and benefits of differing approaches. The modalities include plasmid gene therapeutic, and AAVs. Dilution series, ranges and other aspects of the assay execution are optimized to better fit the best processing paradigm.
Methods: In all cases cells were cultured and maintained per vendor recommendation or established conditions. Flow cytometry was performed using a Cytoflex LX and plates were read using a BioTek SynergyNeo(2). Data was processed using Gen5, PLA or Excel. Dosing of the gene therapy was performed using the nominal established concentrations (vector copy and MOI). Transfection or transduction was facilitated using various agents including Lipofectamine, PEI, Ad5, or other related compounds. Exploratory runs were performed across large concentration and dilution ranges to identify the optimal dosing window. Acquired data was processed for potency determination and comparison made to reference materials and/or controls. Acquisitions were in triplicate throughout.
Results: During de novo assay development dosing curves exploring wide ranges build the foundation for simplified, assay friendly dilution schemes. With the narrower dose range identified the range of the assay can be established next. Factors such as cell health affect the choice of dosing and range. In some instances, higher doses can affect cell health and may be corroborated by visual inspection and/or cytoxicity assays. Approaches such as media exchange following short term treatment, or evaluating lower and fewer dosing points followed post assay approaches such as linear regression analysis can help circumvent toxicity issues leading to robust and reproducible potency determinations. Finally, for therapeutics such as AAV2-like product, approaches outside of ELISA such as flow assays can be implemented to determine expressed protein. The resultant data may be explored using different data treatments owing to variance in the curves and asymptote considerations. As an example, the 4PL and linear analysis provided different levels of “curve robustness”. Additional 5PL evaluation demonstrated that the approach with the highest mathematical rigor (5PL) failed assays unnecessarily while the 4PL and linear evaluations provided comparable performance analytically. Early Phase approaches may utilize matrix tests to establish expression levels and dose ranges, responsive cells lines, before reaching a biological activity indicating assay. Other examples will also be discussed and presented of data treatment for determination of relative potency and accommodation to inherent constraints of a system.
Conclusion: Our data and results demonstrate different approaches to either the development of assays or the processing of data for the determination of relative potency. Finally, we provide recommendations on how to overcome constraints to the analytical system (cells or drugs or both), sensitivities of cells, and the measurement system to finalize an assay suitable for determination of relative potency of a gene therapeutic.
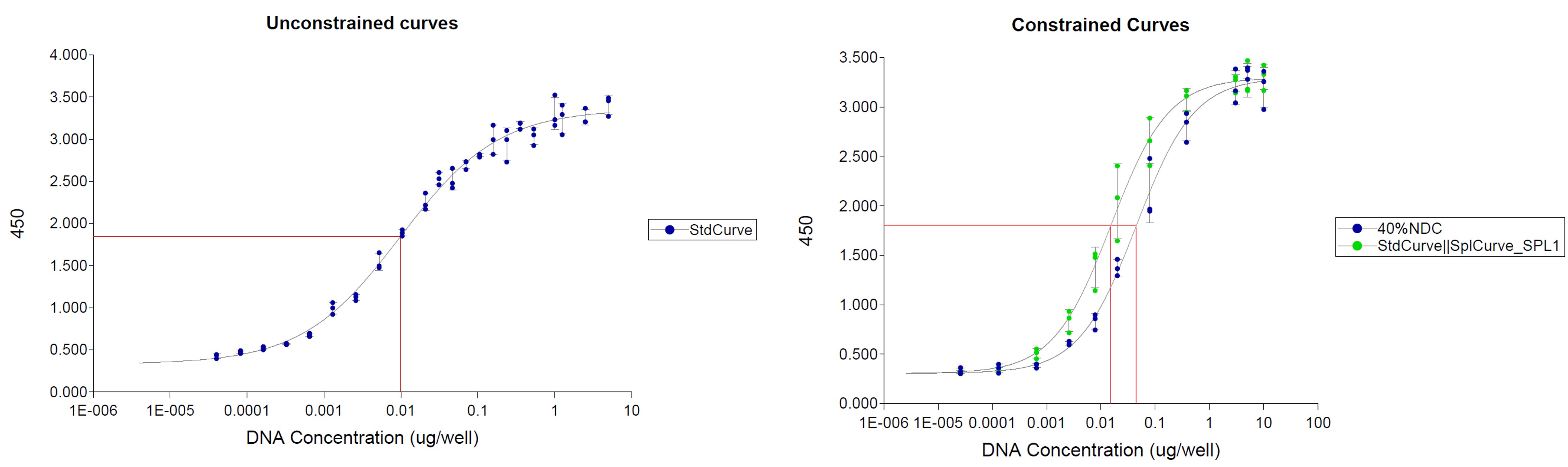 Figure 1: Dose range screening (a) and (b) resultant assay-friendly dilution schemes for a DNA-polymer formulated drug.
Figure 1: Dose range screening (a) and (b) resultant assay-friendly dilution schemes for a DNA-polymer formulated drug.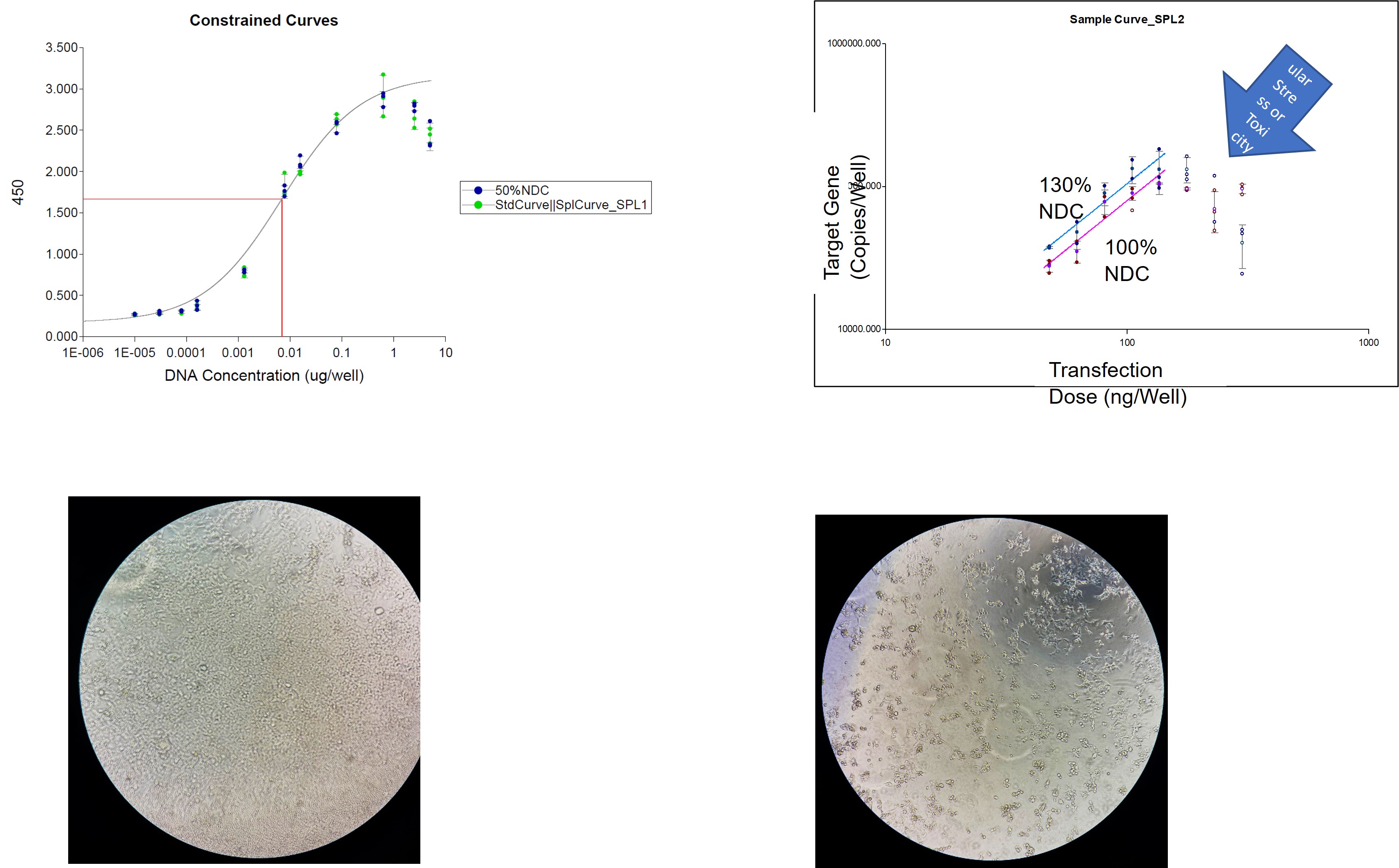 Figure 2: Linear versus 4PL data processing for a Flow based protein expression assay applied to an AAV2-like therapeutic.
Figure 2: Linear versus 4PL data processing for a Flow based protein expression assay applied to an AAV2-like therapeutic. 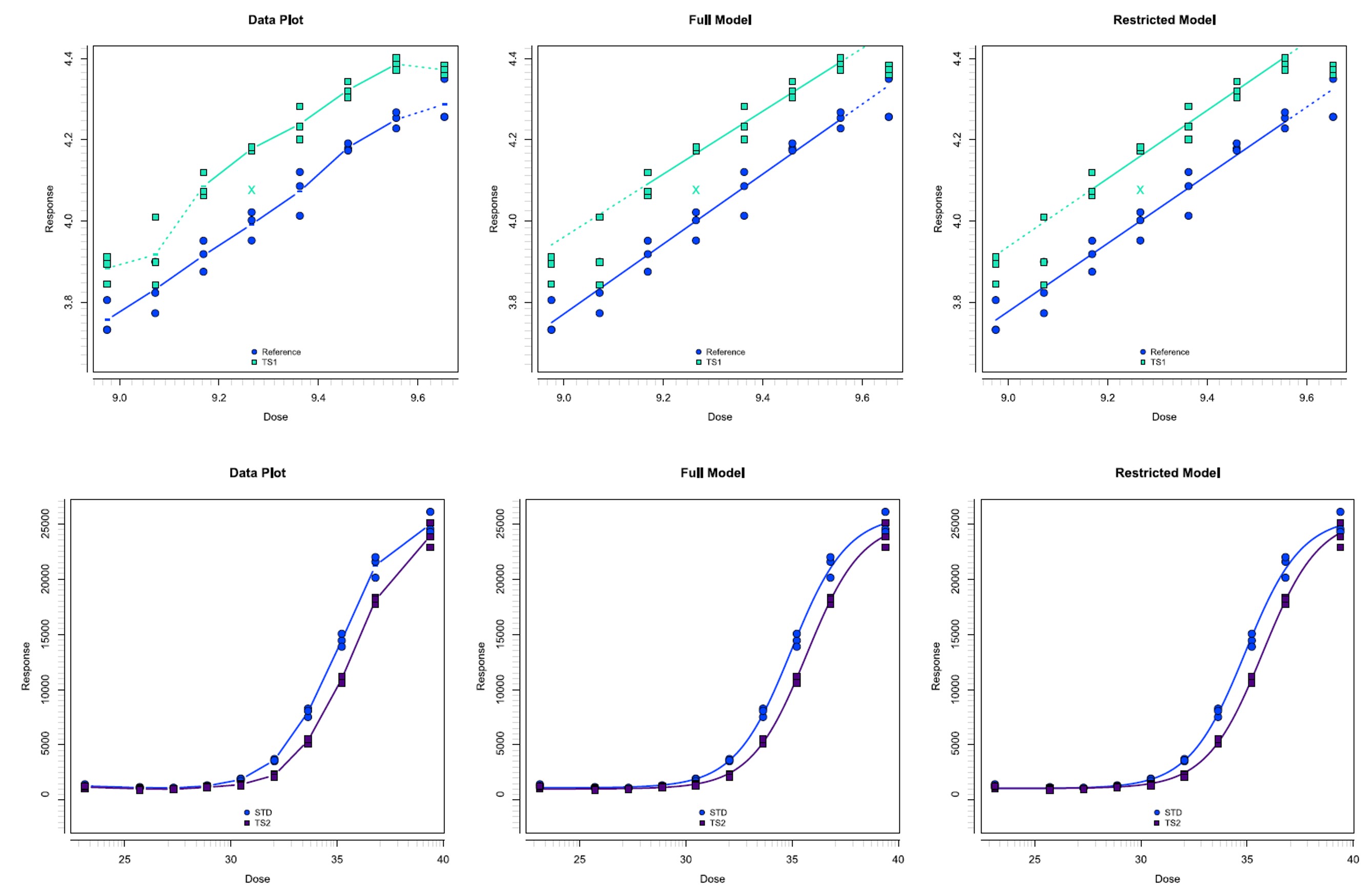 Figure 3: Linear versus 4PL data processing for a Flow based protein expression assay applied to an AAV2-like therapeutic.
Figure 3: Linear versus 4PL data processing for a Flow based protein expression assay applied to an AAV2-like therapeutic.