Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(W0930-12-79) Development of an Extended Release Nafamostat Formulation for Prescription Drug Overdose Protection
- DJ
Dolly Jacob, Ph.D.
Quotient Sciences
Nottingham, England, United Kingdom - VZ
Vanessa Zann, Ph.D.
Quotient Sciences
Nottingham, England, United Kingdom - LK
Lynn Kirkpatrick, Ph.D.
Ensysce Biosciences
La Jolla, California, United States - JM
Jeff Millard, Ph.D.
Ensysce Biosciences
La Jolla, California, United States - LP
Linda Pestano, Ph.D.
Ensysce Biosciences
La Jolla, California, United States - WS
William Schmidt, M.D., Ph.D.
Ensysce Biosciences
La Jolla, California, United States - CE
Cari Evans
Ensysce Biosciences
La Jolla, California, United States - WL
Wu Lin, Ph.D. (he/him/his)
Quotient Sciences
Nottingham, England, United Kingdom - JL
Jeffrey Levy, M.D., Ph.D.
Quotient Sciences
Miami, Florida, United States - KP
Katie Pepper, B.S.
Quotient Sciences
Nottingham, England, United Kingdom
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Prescription opioid abuse and overdose are major societal burdens, resulting in significant costs, illnesses, and deaths. PF614, an oxycodone-derived prodrug, was designed to reduce abuse as it requires exposure to trypsin in the small intestine to release oxycodone. This Trypsin Activated Abuse Protection (TAAPTM) product deters abuse by chemically controlling the rate of release of the active product and preventing activation by all non-oral modes of administration. For added benefit, oral overdose protection (Multi-Pill Abuse Resistance (MPARÒ)) involves combining PF614 with a trypsin inhibitor, nafamostat, in a single dose unit. PF614-MPAR has been developed for potential treatment of moderate-severe pain and is designed to prevent both abuse and overdose. The short half-life of nafamostat required development of an extended release (ER) formulation to ensure trypsin inhibition is maintained for the duration that PF614 prodrug is transiting the small intestine. Both immediate release (IR) and extended release (ER) nafamostat components were evaluated to achieve the desired inhibition of PF614 conversion to oxycodone. A previous multiple ascending dose (MAD) study showed that PF614 25 mg produces oxycodone plasma levels equivalent to 10 mg oxycodone HCl, with peak plasma levels at 6 hours after dosing and a half-life of 11.4 hours in fasted healthy volunteers. The current abstract describes the optimization of the PF614-MPAR formulation with evaluation of several variables including ER nafamostat release rate, ER and IR nafamostat dose ratio, and PF614:nafamostat dose ratio.
Methods: Using the Translational Pharmaceutics platform (rapid manufacturing and clinical testing) allowed iterative optimization of the nafamostat IR/ER formulation based on emerging clinical data. Clinically, different release rates of the ER beads and IR/ER dose combinations were assessed by evaluating the pharmacokinetics (PK) of oxycodone release from PF614. The goal was to identify a unit dose of nafamostat (IR and/or ER) which didn’t impact oxycodone release from PF614 when the prescribed dose was ingested (1 or 2 units) but would inhibit PF614 activation in an overdose situation (3 or more units). A 2-dimensionl design space for the ER beads (Figure 1), varying both nafamostat dose and release rate, was combined with a dose bracket for the IR nafamostat solution. Nafamostat ER beads were developed using methacrylate copolymers with release rates targeting a cumulative 90% release in 4 to 16 hours and a dose range of 0.25 mg to 35 mg, with a maximum dose used clinically of 10 mg. ER beads were filled into capsules for dosing and were co-administered with PF614 and nafamostat IR oral solutions. The clinical study was a 2-part, randomized, open-label study. Part A was a formulation development study for the nafamostat formulation (IR solution and/or ER prototype capsules). Part B assessed PF614 25 mg combined with the formulated nafamostat prototype selected from Part A, in increasing dose units from 1 to 8 capsules administered simultaneously to simulate opioid overdose in naltrexone-blocked healthy volunteers. Part B also assessed twice a day dosing with the selected formulation in healthy subjects.
Results: 11 cohorts of 6 or 8 healthy volunteers were dosed with PF614 25 mg alone or with various IR/ER nafamostat combinations in Part A. ER nafamostat beads with release rates of 90% at 9 hours or 90% at 6 hours were evaluated. The effect of 1 to 10 mg nafamostat (IR and ER combination) on oxycodone release was assessed. A nafamostat formulation with the optimal ER release rate, ratio of IR and ER nafamostat, and total dose for the PF614-MPAR 25 mg dose unit was identified in Part A (Figure 2). Part B assessed the selected unit dose [PF614 25 mg and 1 mg formulated nafamostat (IR/ER)] in an overdose situation by assessing oxycodone exposure after 1, 2-, 3-, 5-, or 8-unit doses were administered simultaneously. Exposure of oxycodone with 1 or 2 dose units produced oxycodone plasma levels similar to doses of PF614 25 mg or 50 mg alone, showing that the addition of nafamostat had no effect on PF614 conversion to oxycodone. However, with simultaneous administration of 3 units or greater, oxycodone plasma levels were reduced compared to PF614 alone at the same dose levels in prior single and multiple ascending dose (MAD) studies, confirming inhibition of PF614 conversion to oxycodone (Figure 3). The study aim of identifying a PF614-MPAR (nafamostat containing) formulation that allowed PF614 conversion at 1 to 2 dose units but that inhibited trypsin conversion to oxycodone when administered in a simulated overdose situation was achieved.
Conclusion: This study defined a PF614-MPAR (25 mg) single unit dose that delivered oxycodone at a prescribed plasma level equivalent to 10 mg oxycodone HCl but prevents oral overdose when consumed in excess. A proof-of-concept arm, dosing increasing unit doses of PF614 combined with formulated nafamostat (PF614-MPAR), provided evidence that MPAR effectively attenuated oxycodone levels at up to 8x higher dose levels compared to PF614 alone, supporting its provision of overdose protection.
References: Kirkpatrick DL et al. Clinical evaluation of PF614, a novel TAAP(TM) prodrug of oxycodone, versus OxyContin® in a multi-ascending dose study with a bioequivalence arm in healthy volunteers. Submitted, 2013.
Acknowledgements: Sponsorship by the National Institute on Drug Abuse is gratefully acknowledged (5UH3DA047682-04). Clinical protocol reviewed and approved by Advarra IRB (MOD01165092; 3 Dec 2021).
.jpg) Figure 1: Schematic of the 2D design space
Figure 1: Schematic of the 2D design space 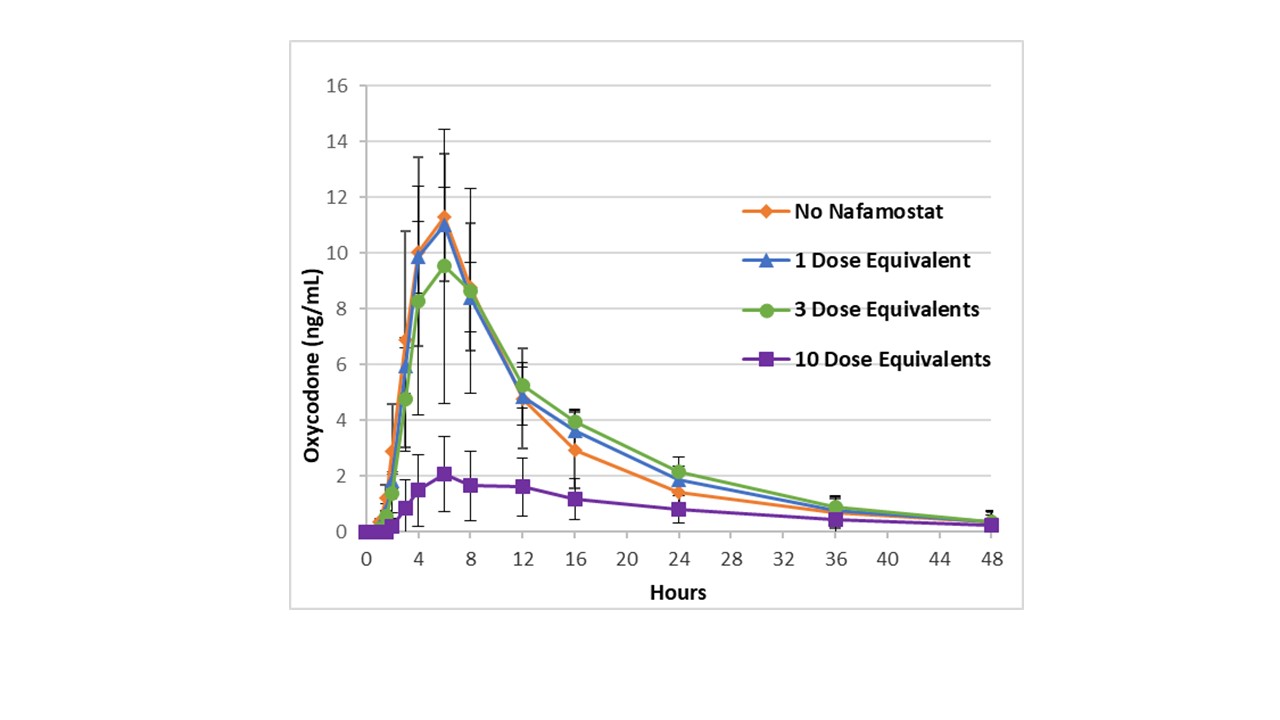 Figure 2: Plasma concentration time profiles of oxycodone released from PF614 following oral administration of PF614 (25 mg) with 1X, 3X or 10X dose of nafamostat IR/ER formulation (1 mg)
Figure 2: Plasma concentration time profiles of oxycodone released from PF614 following oral administration of PF614 (25 mg) with 1X, 3X or 10X dose of nafamostat IR/ER formulation (1 mg)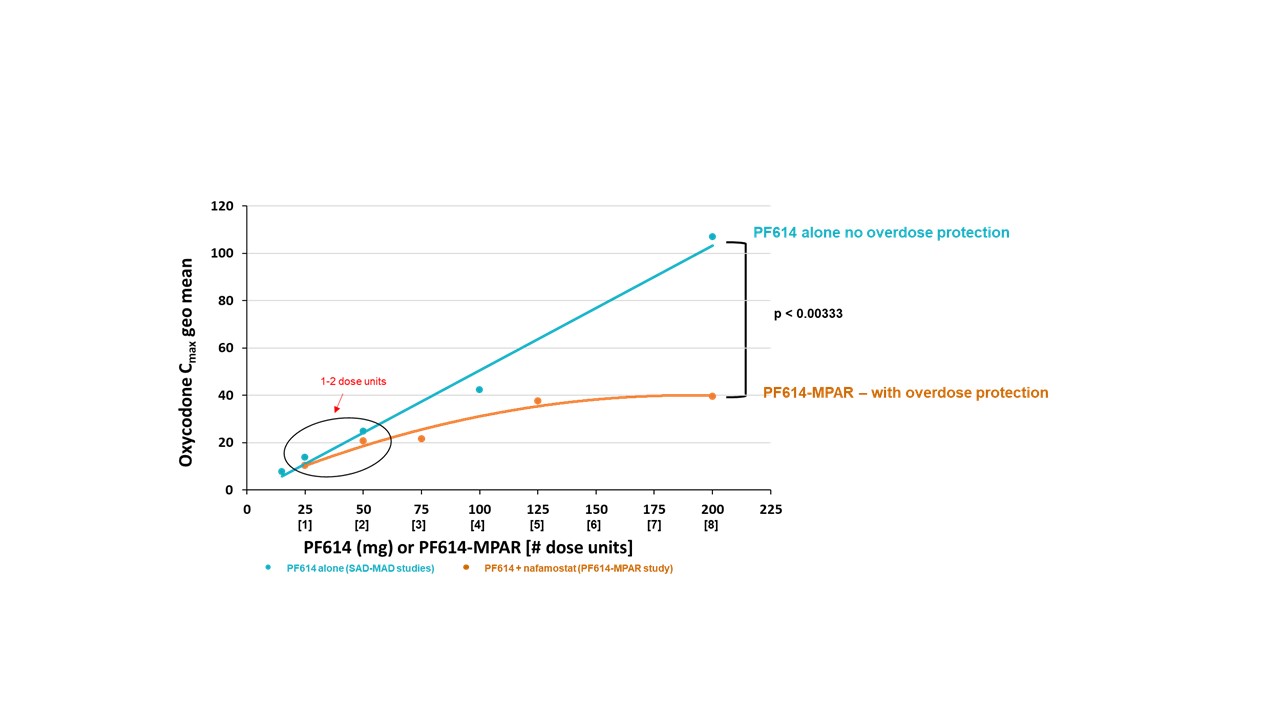 Figure 3: Oxycodone Cmax levels when administered with increasing unit dose levels or PF614 alone or PF614 and nafamostat IR/ER formulation
Figure 3: Oxycodone Cmax levels when administered with increasing unit dose levels or PF614 alone or PF614 and nafamostat IR/ER formulation