Discovery and Basic Research
Category: Late Breaking Poster Abstract
(W0930-05-32) Repurposing an Antiviral Peptide to Inhibit Tumor-Derived Exosomes for Synergistic Cancer Immunotherapy
Wednesday, October 25, 2023
9:30 AM - 10:30 AM ET
- SS
Sol Shin
SungKyunKwan University
Suwon, Kyonggi-do, Republic of Korea - SS
Sol Shin
SungKyunKwan University
Suwon, Kyonggi-do, Republic of Korea - HK
Hyewon Ko
Korea Research Institute of Bioscience and Biotechnology
Suwon, Kyonggi-do, Republic of Korea - JJ
Joshua A. Jackman
SungKyunKwan University
Suwon, Kyonggi-do, Republic of Korea - JP
Jae Hyung Park, Ph.D.
SungKyunKwan University
Suwon, Kyonggi-do, Republic of Korea
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Tumor-derived exosomes (T-EXOs) are implicated in conferring resistance to immune checkpoint blockade (ICB) therapy, which has motivated efforts to inhibit T-EXO biogenesis. Inspired by how a membrane-curvature-sensing, antiviral peptide can directly inhibit membrane-enveloped viruses, we developed a strategy, termed Lipid Envelope Exosome Disruption (LEED), that is based on repurposing such peptides to directly inhibit membrane-enveloped T-EXOs for synergistic cancer immunotherapy. The peptide inhibits T-EXOs originating from various cancer cell types and peptide-mediated membrane disruption of T-EXOs is enhanced under acidic pH conditions found in tumor microenvironments. The combination of T-EXO-disrupting peptide therapy and programmed cell death protein-1 (PD-1) antibody-based ICB therapy led to improved cancer treatment outcomes in a tumor-bearing mouse model compared to antibody therapy alone. Peptide-mediated disruption of T-EXOs not only reduced levels of circulating exosomal programmed death-ligand 1 (PD-L1), but also restored CD8+ T cell effector function, prevented the formation of pre-metastatic niches, and reshaped the tumor microenvironment in vivo. Our findings demonstrate that the LEED strategy can enhance cancer immunotherapy and support the potential of peptide engineering for exosome targeting applications.
Methods: In this study, to realize the LEED strategy, we utilized a 27-mer amphipathic, α-helical peptide known as AH-D, which has potent membrane-disrupting and antiviral properties. We also designed a control peptide (NH-D) with specific mutations to disrupt its helical and amphipathic properties. To confirm the secondary structures of both peptides, circular dichroism spectroscopy experiments were perfomed. We used the quartz crystal microbalance-dissipation (QCM-D) technique to assess the ability of AH-D to rupture surface-adsorbed liposomes, which mimic membrane-enveloped exosomes and conducted QCM-D experiments to examine the rupture activity of AH-D under different pH conditions resembling the tumor microenvironment. Additionally, transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and western blot analysis were used to verify the effects of AH-D on authentic T-EXOs derived from melanoma cells. Enzyme-linked immunosorbent assay (ELISA) and confocal microscopy were employed to investigate the binding capacity of T-EXOs to PD-1 proteins and their cellular uptake by CD8+ T cells, respectively. Flow cytometry experiments were conducted to evaluate CD8+ T cell proliferation and their cytotoxic functions after exposure to T-EXOs treated with AH-D. Finally, we also assessed the in vivo effects of AH-D treatment on the immunosuppressive tumor microenvironment and circulating levels of PD-L1-expressing exosomes in melanoma-bearing mice.
Results: The results demonstrated that AH-D peptide effectively ruptured surface-adsorbed liposomes, indicating its membrane-disrupting activity. The extent of membrane fusion induced by AH-D was more significant under acidic pH conditions. AH-D treatment also induced fusion-mediated rupture of authentic T-EXOs, leading to reduced binding capacity to PD-1 proteins and diminished cellular uptake by CD8+ T cells. Consequently, AH-D treatment inhibited T-EXO-mediated T cell dysfunction and enhanced the proliferative potential and cytotoxic functions of CD8+ T cells. In vivo studies showed that AH-D peptide reshaped the tumor microenvironment by reducing immunosuppressive cell populations and decreasing the levels of circulating PD-L1-expressing exosomes. Combination therapy of AH-D peptide with an anti-PD-1 inhibitor (aPD-1) exhibited a significant reduction in tumor growth and metastasis compared to aPD-1 monotherapy.
Conclusion: In this study, we devised the LEED strategy that poses a new direction to deplete T-EXO effectively. As a first attempt to prove the LEED strategy, we harnessed the antiviral AH-D peptide capable of recognizing high membrane curvature. The AH-D peptide induced a rapid physical disruption of T-EXOs, which counteracted the immunosuppressive effect of exosomal PD-L1. Combination therapy of AH-D peptide with aPD-1 showed enhanced anti-tumor efficacy by reshaping the tumor microenvironment and preventing the formation of pre-metastatic niches. The findings highlight the potential of using AH-D peptide to enhance the therapeutic outcome of immune checkpoint blockade therapy and provide insights into the mechanisms underlying the antitumor effects of exosome-targeting strategies.
References: [1] Tang, J. et al. The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat. Rev. Drug Discovery 17, 854-855 (2018).
[2] Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350-1355 (2018).
[3] Sharma, P. et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707-723 (2017).
[4] Sun, C. et al. Regulation and function of the PD-L1 checkpoint. Immunity 48, 434-452 (2018).
[5] Cha, J.-H. et al. Mechanisms controlling PD-L1 expression in cancer. Molecular Cell 76, 359-370 (2019).
[6] Daassi, D. et al. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 20, 209-215 (2020).
[7] Poggio, M. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177, 414-427.e413 (2019).
[8] Yang, Y. et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 28, 862-864 (2018).
[9] Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382-386 (2018).
[10] Zhang, C. et al. Anti-PD-1 therapy response predicted by the combination of exosomal PD-L1 and CD28. Front. Oncol. 10, 760 (2020).
[11] Del Re, M. et al. Blood-based PD-L1 analysis in tumor-derived extracellular vesicles: Applications for optimal use of anti-PD-1/PD-L1 axis inhibitors. Biochim. Biophys. Acta, Rev. Cancer 1875, 188463 (2021).
[12] Pegtel, D. M. & Gould, S. J. Exosomes. Annu. Rev. Biochem. 88, 487-514 (2019).
[13] Marar, C. et al. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 22, 560-570 (2021).
[14] Xie, F. et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv. Sci. 6, 1901779 (2019).
[15] Zhang, H. et al. Advances in the discovery of exosome inhibitors in cancer. J. Enzyme Inhib. Med. Chem. 35, 1322-1330 (2020).
[16] Kwon, S. et al. Engineering approaches for effective therapeutic applications based on extracellular vesicles. J. Controlled Release 330, 15-30 (2021).
[17] Van Niel, G et al. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213-228 (2018).
[18] Catalano, M. & O'Driscoll, L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J. Extracell. Vesicles 9, 1703244 (2020).
[19] Nolte-‘t Hoen, E et al. Extracellular vesicles and viruses: are they close relatives? Proc. Natl. Acad. Sci. 113, 9155-9161 (2016).
[20] Jackman, J. A. et al. Therapeutic treatment of Zika virus infection using a brain-penetrating antiviral peptide. Nat. Mater. 17, 971-977 (2018).
[21] Jackman, J. A. et al. Targeting the Achilles heel of mosquito-borne viruses for antiviral therapy. ACS Infect. Dis. 5, 4-8 (2019).
[22] De Lázaro, I. & Mooney, D. J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. (2021)
[23] Camargos, V. N. et al. In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy. EBioMedicine 44, 516-529 (2019).
[24] Cho, N.-J. et al. Mechanism of an amphipathic α-helical peptide’s antiviral activity involves size-dependent virus particle lysis. ACS Chem. Biol. 4, 1061-1067 (2009).
[25] Park, S. et al. Comparing the membrane-interaction profiles of two antiviral peptides: insights into structure–function relationship. Langmuir 35, 9934-9943 (2019).
[26] Christensen, C. et al. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site-specifically labelled PD-L1 antibody. Eur. J. Nucl. Med. Mol. Imaging 47, 1302-1313 (2020).
[27] Jackman, J. A. et al. Rupture of lipid vesicles by a broad-spectrum antiviral peptide: influence of vesicle size. J. Phys. Chem. B 117, 16117-16128 (2013).
[28] Jackman, J. A. et al. Deciphering how pore formation causes strain-induced membrane lysis of lipid vesicles. J. Am. Chem. Soc. 138, 1406-1413 (2016).
[29] Snider, C et al. MPEx: a tool for exploring membrane proteins. Protein Sci. 18, 2624-2628 (2009).
[30] Badani, H. et al. Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. Biochim. Biophys. Acta, Biomembr. 1838, 2180-2197 (2014).
[31] Gunasekaran, M. et al. Exosomal PD-L1 expression as non-invasive biomarker for immune checkpoint inhibitors in non-small cell lung cancer. J. Immunol. 204, 90.10 (2020).
[32] Batlle, E. & Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924-940 (2019).
[33] Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544-548 (2018).
[34] Guha, S et al. Mechanistic landscape of membrane-permeabilizing peptides. Chem. Rev. 119, 6040-6085 (2019).
[35] Furukawa, N. & Popel, A. S. Peptides that immunoactivate the tumor microenvironment. Biochim. Biophys. Acta, Rev. Cancer 1875, 188486 (2021).
[36] Webber, J. et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70, 9621-9630 (2010).
[37] Chen, X. & Song, E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat. Rev. Drug Discovery 18, 99-115 (2019).
[38] Kato, T. et al. Cancer-associated fibroblasts affect intratumoral CD8+ and FoxP3+ T cells via IL6 in the tumor microenvironment. Clin. Cancer Res. 24, 4820-4833 (2018).
[39] Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329-335 (2015).
[40] Ortiz, A. et al. An interferon-driven oxysterol-based defense against tumor-derived extracellular vesicles. Cancer Cell 35, 33-45.e36 (2019).
[41] Lu, Z. et al. Regulation of intercellular biomolecule transfer–driven tumor angiogenesis and responses to anticancer therapies. J. Clin. Investig. 131, e144225 (2021).
[42] Murgai, M. et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat. Med. 23, 1176-1190 (2017).
[43] Ji, Q. et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat. Commun. 11, 1211 (2020).
[44] Grum-Schwensen, B. et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res. 65, 3772-3780 (2005).
[45] Fang, T. et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 9, 191 (2018).
[46] O'Connell, J. T. et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. 108, 16002-16007 (2011).
[47] Vitale, I. et al. Targeting cancer heterogeneity with immune responses driven by oncolytic peptides. Trends Cancer 7, 557-572 (2021).
[48] Rodrigues, G. et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 21, 1403-1412 (2019).
[49] Gao, L. et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 19, 233-245 (2018)
Woo, C. H. et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 9, 1735249 (2020).
[50] Woo, C. H. et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles 9, 1735249 (2020).
[51] Jackman, J. A. et al. Vesicle adhesion and rupture on silicon oxide: Influence of freeze–thaw pretreatment. Langmuir 30, 2152-2160 (2014).
[52] Di Nardo, G. et al. Evidence for an elevated aspartate pKa in the active site of human aromatase. J. Biol. Chem. 290, 1186-1196 (2015).
[53] Son, S. et al. Repurposing macitentan with nanoparticle modulates tumor microenvironment to potentiate immune checkpoint blockade. Biomaterials 276, 121058 (2021).
Acknowledgements:This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (No. PS-2023-00256265), by the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (No. HN22C0624).
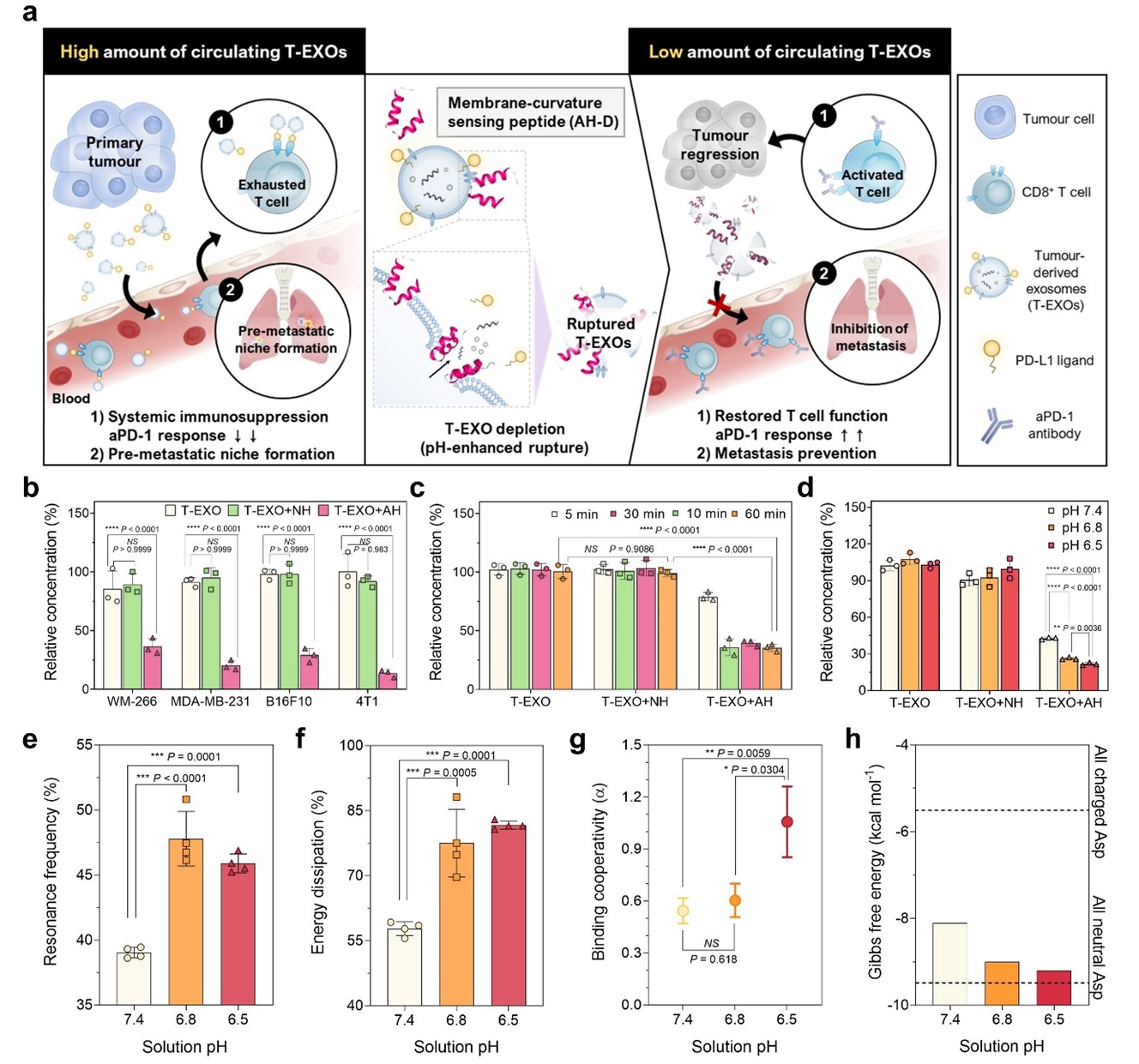 Fig. 1 | Repurposed antiviral AH-D peptide disrupts T-EXOs with enhanced activity in tumor pH conditions. a, Illustration of T-EXO depletion strategy. b, Nanoparticle tracking analysis (NTA) change in normalized number concentration of T-EXOs from human and murine cancer cells following T-EXO incubation with 1 μM AH-D or NH-D peptide for 10 min. WM-266, MDA-MB-231, B16F10, and 4T1 are human melanoma, human breast cancer, murine melanoma, and murine breast cancer cell lines, respectively. c-d, Corresponding NTA results for B16F10-derived T-EXOs following incubation with 1 μM AH-D or NH-D peptide for different time intervals (c) or for 5 min in different pH conditions (d). Results in b-d are reported as mean ± standard deviation (s.d.) (n=3 biological replicates, one-way analysis of variance (ANOVA)). e-f, Quartz crystal microbalance-dissipation (QCM-D) maximal changes in resonance frequency (e) and energy dissipation (f) signals following addition of 32 μM AH-D peptide to a layer of surface-adsorbed liposomes in different pH conditions. Results in e-f are reported as mean ± s.d. (n=4 biological replicates, one-way ANOVA). g, Binding cooperativity of AH-D peptide-induced liposomal membrane rupture in different pH conditions. Results are reported as best-fit values ± s.d. from least squares regression (n=16 biological replicates, extra sum-of-squares F tests between groups). h, Change in Gibbs free energy for membrane partitioning of AH-D peptide in different pH environments. Modelling is based on the Wimley-White interfacial hydrophobicity scale and dashed lines correspond to all charged (top) or all neutral (bottom) Asp residues in the peptide.
Fig. 1 | Repurposed antiviral AH-D peptide disrupts T-EXOs with enhanced activity in tumor pH conditions. a, Illustration of T-EXO depletion strategy. b, Nanoparticle tracking analysis (NTA) change in normalized number concentration of T-EXOs from human and murine cancer cells following T-EXO incubation with 1 μM AH-D or NH-D peptide for 10 min. WM-266, MDA-MB-231, B16F10, and 4T1 are human melanoma, human breast cancer, murine melanoma, and murine breast cancer cell lines, respectively. c-d, Corresponding NTA results for B16F10-derived T-EXOs following incubation with 1 μM AH-D or NH-D peptide for different time intervals (c) or for 5 min in different pH conditions (d). Results in b-d are reported as mean ± standard deviation (s.d.) (n=3 biological replicates, one-way analysis of variance (ANOVA)). e-f, Quartz crystal microbalance-dissipation (QCM-D) maximal changes in resonance frequency (e) and energy dissipation (f) signals following addition of 32 μM AH-D peptide to a layer of surface-adsorbed liposomes in different pH conditions. Results in e-f are reported as mean ± s.d. (n=4 biological replicates, one-way ANOVA). g, Binding cooperativity of AH-D peptide-induced liposomal membrane rupture in different pH conditions. Results are reported as best-fit values ± s.d. from least squares regression (n=16 biological replicates, extra sum-of-squares F tests between groups). h, Change in Gibbs free energy for membrane partitioning of AH-D peptide in different pH environments. Modelling is based on the Wimley-White interfacial hydrophobicity scale and dashed lines correspond to all charged (top) or all neutral (bottom) Asp residues in the peptide.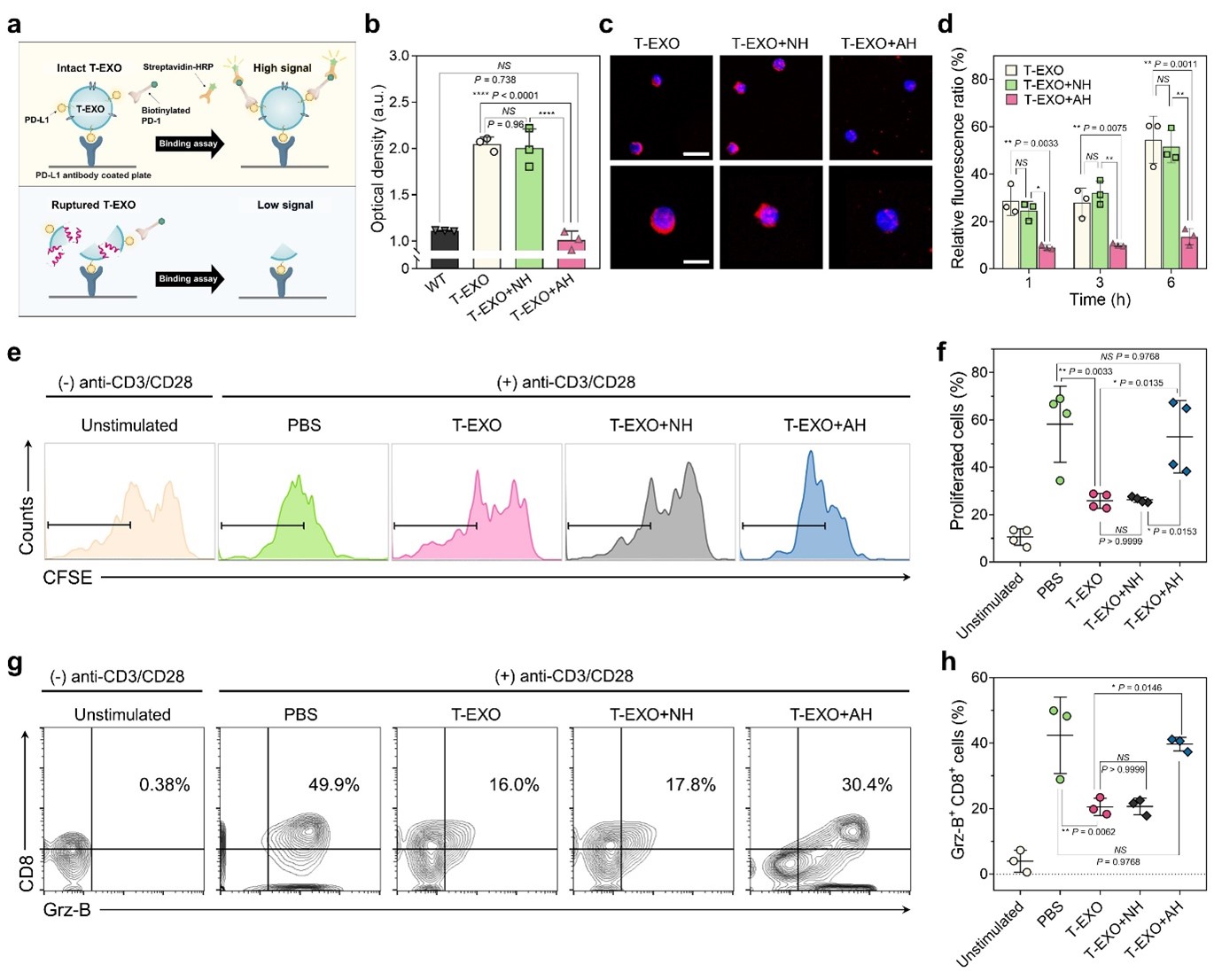 Fig. 2 | AH-D peptide prevents T-EXO-mediated CD8+ T cell dysfunction in vitro. a-b, PD-1 binding to exosomal PD-L1 by enzyme-linked immunosorbent assay (ELISA). Measurement concept (a) and PD-1 binding levels (b) to T-EXOs pretreated with AH-D or NH-D peptide (n=3 biological replicates, one-way ANOVA). T-EXOs and control exosomes were isolated from the plasma of B16F10 tumor-bearing and wild-type (WT) mice, respectively. c-h, Splenic naïve CD8+ T cells were stimulated with anti-CD3/CD28 antibodies. T-EXOs, without or with AH-D or NH-D peptide pretreatment, were added to stimulated T cells, followed by microscopy or flow cytometry analysis. Confocal microscopy images (c) of T cells after 3 h incubation with T-EXOs. Scale bars, 20 μm (upper) and 10 μm (lower). Blue, cell nuclei; Red, Cy5.5 dye-labelled T-EXOs. The images are representative of four independent experiments. Relative fluorescence intensity values (d) of T-EXO uptake by T cells at different time intervals. Representative flow cytometry plots (e) showing carboxyfluorescein succinimidyl ester (CFSE) dilution of CD8+ T cells and proportion (f) of CFSE-labelled CD8+ T cells. Representative flow cytometry plots after gating (g) and proportion (h) of activated CD8+ T cells that express granzyme B (Grz-B). Results in d, f, and h are reported as mean ± s.d. (n=3-4 biological replicates, one-way ANOVA).
Fig. 2 | AH-D peptide prevents T-EXO-mediated CD8+ T cell dysfunction in vitro. a-b, PD-1 binding to exosomal PD-L1 by enzyme-linked immunosorbent assay (ELISA). Measurement concept (a) and PD-1 binding levels (b) to T-EXOs pretreated with AH-D or NH-D peptide (n=3 biological replicates, one-way ANOVA). T-EXOs and control exosomes were isolated from the plasma of B16F10 tumor-bearing and wild-type (WT) mice, respectively. c-h, Splenic naïve CD8+ T cells were stimulated with anti-CD3/CD28 antibodies. T-EXOs, without or with AH-D or NH-D peptide pretreatment, were added to stimulated T cells, followed by microscopy or flow cytometry analysis. Confocal microscopy images (c) of T cells after 3 h incubation with T-EXOs. Scale bars, 20 μm (upper) and 10 μm (lower). Blue, cell nuclei; Red, Cy5.5 dye-labelled T-EXOs. The images are representative of four independent experiments. Relative fluorescence intensity values (d) of T-EXO uptake by T cells at different time intervals. Representative flow cytometry plots (e) showing carboxyfluorescein succinimidyl ester (CFSE) dilution of CD8+ T cells and proportion (f) of CFSE-labelled CD8+ T cells. Representative flow cytometry plots after gating (g) and proportion (h) of activated CD8+ T cells that express granzyme B (Grz-B). Results in d, f, and h are reported as mean ± s.d. (n=3-4 biological replicates, one-way ANOVA).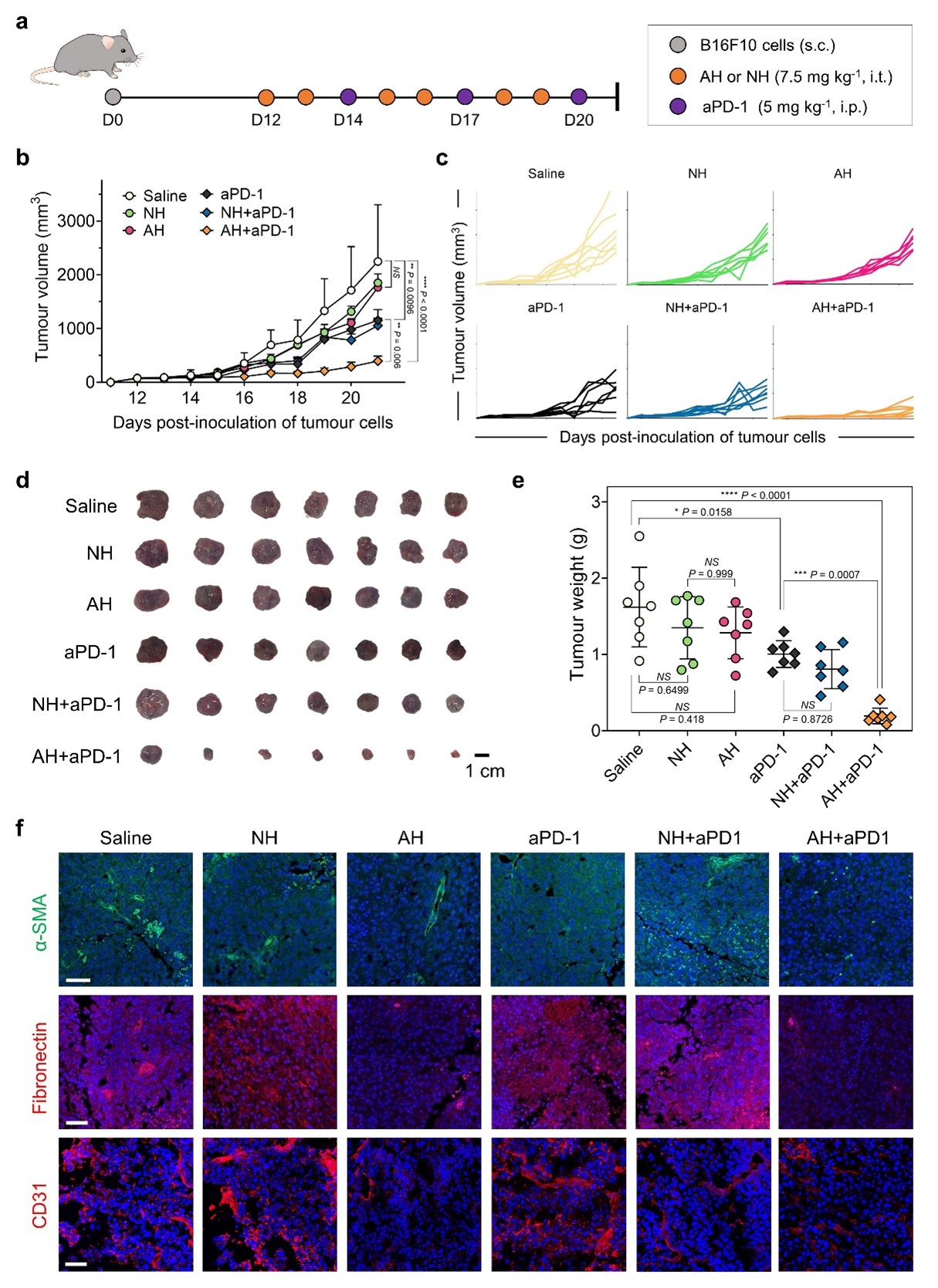 Fig. 3 | AH-D peptide enhances aPD-1 antibody therapy and reshapes tumor microenvironment in mice. a, B16F10 tumor-bearing C57BL/6 mice were treated with AH-D or NH-D peptide (7.5 mg kg-1 intratumoral), aPD-1 antibody (5 mg kg-1 intraperitoneal), or combinations thereof on selected days post-inoculation. b, Tumor volumes were measured starting day 12 post-inoculation and results are reported as mean ± s.d. (n=7 mice per group, one-way ANOVA). c, Tumor volumes of individual mice corresponding to the data in b. d-e, Representative pictures (d) and weights (e) of the excised tumors. Results in e are reported as mean ± s.d. (n=7 mice per group, one-way ANOVA). f, Immunofluorescence microscopy images of α-SMA, fibronectin, and CD31 in tumor tissues. Scale bar, 50 μm. Blue, cell nuclei. The images are representative of four independent experiments.
Fig. 3 | AH-D peptide enhances aPD-1 antibody therapy and reshapes tumor microenvironment in mice. a, B16F10 tumor-bearing C57BL/6 mice were treated with AH-D or NH-D peptide (7.5 mg kg-1 intratumoral), aPD-1 antibody (5 mg kg-1 intraperitoneal), or combinations thereof on selected days post-inoculation. b, Tumor volumes were measured starting day 12 post-inoculation and results are reported as mean ± s.d. (n=7 mice per group, one-way ANOVA). c, Tumor volumes of individual mice corresponding to the data in b. d-e, Representative pictures (d) and weights (e) of the excised tumors. Results in e are reported as mean ± s.d. (n=7 mice per group, one-way ANOVA). f, Immunofluorescence microscopy images of α-SMA, fibronectin, and CD31 in tumor tissues. Scale bar, 50 μm. Blue, cell nuclei. The images are representative of four independent experiments.