Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(T1530-09-58) Exploring Applications of Plant-Derived Polymers in Fused Deposition Modeling of Oral Pharmaceutical Tablets
Tuesday, October 24, 2023
3:30 PM - 4:30 PM ET
.jpg)
Talles Ernica, Ph.D.
Global Pharma Technical Developer
Roquette America Inc.
PHILADELPHIA, Pennsylvania, United States- KC
Keat Theng Chow, Ph.D.
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore - HG
Hui Ping Goh, Ph.D.
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore - YL
Yuan Siang Liu, Ph.D.
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore - NC
Nicholas Chua, B.A.
Roquette Asia Pacific Pte. Ltd.
Singapore, Singapore - OH
Olaf Haeusler, Ph.D.
Roquette Pte., Ltd.
Lestrem, Nord-Pas-de-Calais, France - AB
Antoine Billa, Ph.D.
Roquette Pte., Ltd.
Lestrem, Nord-Pas-de-Calais, France - MA
Marie Albert, Ph.D.
Roquette Pte., Ltd.
Lestrem, Nord-Pas-de-Calais, France
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Additive manufacturing or 3D printing technology refers to the fabrication of three-dimensional objects from computer-aided designs (CAD) by adding layers of material on top of each other successively. In oral pharmacotherapy, this technology presents a paradigm shift from the current “one size fits all” approach. The 3D printing technology will allow the manufacture of solid dosage forms with different dose strengths, combinations of different medicaments or drug release profiles. To achieve these properties, the 3D printing technology will work by altering the size, shape and fill level of the tablet directly, which cannot be attained easily with conventional manufacturing methods. Fused deposition modeling (FDM) is one of the most investigated 3D printing technologies for oral tablets. While synthetic oil-based materials such as polyvinylpyrrolidone, polyvinyl alcohol, and polylactic acid are commonly employed in FDM formulations, there is currently limited information on the applicability of safer and more sustainable plant-based alternatives. The purpose of this study was to evaluate the suitability of modified starches in 3D printing of immediate and controlled release tablets containing a model active pharmaceutical ingredient (API) via FDM technology.
Methods: Diprophylline was used as the model API. Prior to FDM printing of the tablets, filaments were prepared via hot melt extrusion (HME) using a twin screw extruder fitted with a 2.6 mm die orifice. Sorbitol (NEOSORB® 100 C) and mannitol (PEARLITOL® 100 SD) were included as plasticizers in the formulations while stearic acid was utilized as the lubricant. Pregelatinized hydroxypropyl pea starch (LYCOAT® RS 720) was used as the polymer matrix in Formulation 1 while a combination of pregelatinized potato starch (PREGEFLO® PI 10) and hydroxypropyl methylcellulose (HPMC K4M) were used in Formulation 2 to achieve immediate release and controlled release profile respectively with the model API. Critical HME process parameters such as screw speed and barrel temperature were controlled. A mixture design approach was used to vary formulation components to investigate effects of plasticizer ratio, starch and stearic acid concentration on filament properties and tablet printability. Flat cylindrical tablets of 10 mm diameter and 5 mm thickness were printed from optimized formulations using a benchtop 3D printer. Dissolution studies using USP Apparatus 2 were performed for both immediate (Formulation 1) and controlled-release (Formulation 2) tablets for 2-hour and 12-hour duration, respectively. Simulated gastric fluid (SGF pH 1.2) and simulated intestinal fluid (SIF pH 6.8) without enzymes were used as the dissolution mediums to mimic fasted conditions. Drug assay was done via UV spectrophotometry at a wavelength of 272 nm. Tablets were also assessed for their stability by storing them in heat-sealed aluminum pouches under two conditions (25°C / 60% relative humidity and 40°C / 75% relative humidity) for at least one month.
Results: Sorbitol had stronger plasticizer effect than mannitol, and optimal ratios of sorbitol to mannitol were found for immediate and controlled release formulations to create the balance between filament brittleness and pliability. Stearic acid had some impact on filament brittleness even at low concentrations as a lubricant and anti-tacking agent. Formulations with high starch content but low HPMC concentration yielded brittle filaments which were not suitable for further processing. Formulations devoid of starch on the other hand were not extrudable. Optimized filament formulations could be extruded continuously and be used for FDM 3D printing. Up to 84.2 % of the API was released by 30 minutes in the immediate release formulations while 74.7 % of the API was released by 8 hours in the controlled release formulation. As a result, immediate release and controlled release formulations both fulfill FDA requirements for drug release. Dissolution profiles remain unchanged after 1 month of storage.
Conclusion: It was demonstrated that modified starches were useful for HME and FDM 3D printing. In particular, the combination of pregelatinized starch and HPMC yielded extrudable filaments capable of controlled drug release when printed into tablets. Plant-based polymers could prove to be useful and safer alternatives to conventional polymers used in additive manufacturing of oral tablets.
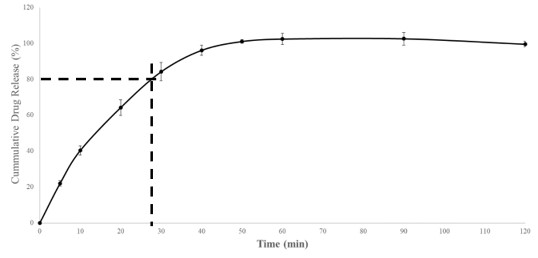 1. Dissolution profile of immediate release diprophylline tablets tested using USP Apparatus 2 in SGF pH 1.2 media without enzymes.
1. Dissolution profile of immediate release diprophylline tablets tested using USP Apparatus 2 in SGF pH 1.2 media without enzymes.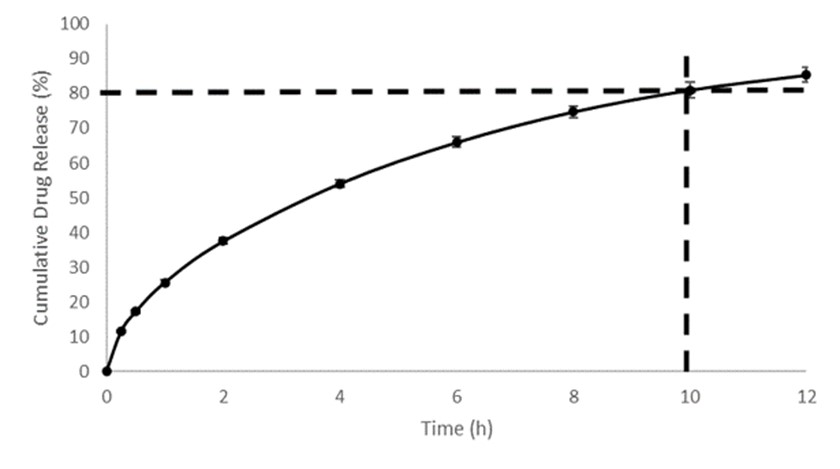 2. Dissolution profile of controlled release diprophylline tablets tested using USP Apparatus 2 in SGF-SIF pH transition media without enzymes.
2. Dissolution profile of controlled release diprophylline tablets tested using USP Apparatus 2 in SGF-SIF pH transition media without enzymes.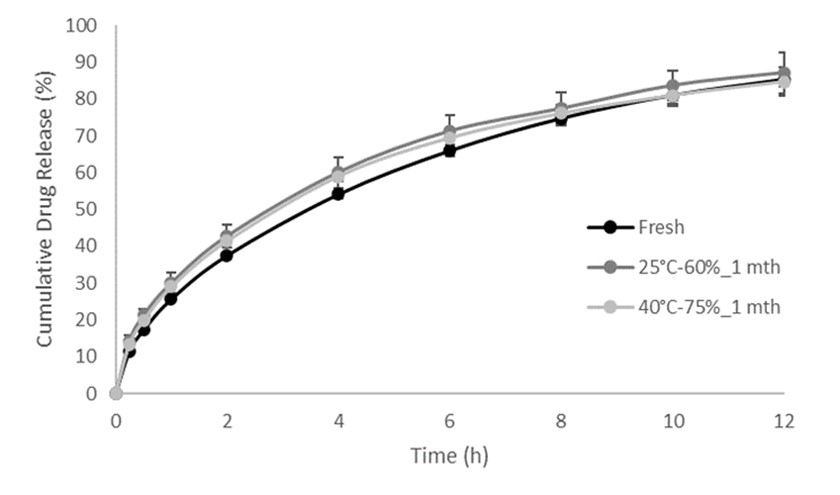 3. Dissolution profile comparison of controlled release diprophylline tablets after 1 month stability at 25°C / 60% RH and 40°C / 75% RH, tested using USP Apparatus 2 in SGF-SIF pH transition media without enzymes.
3. Dissolution profile comparison of controlled release diprophylline tablets after 1 month stability at 25°C / 60% RH and 40°C / 75% RH, tested using USP Apparatus 2 in SGF-SIF pH transition media without enzymes.