Formulation and Delivery - Biomolecular
Category: Late Breaking Poster Abstract
(T1230-03-16) Conjugation Chemistry of Antibody-Liposome Conjugates Affects Performance via Complement Activation and Mechanisms Thereof
Tuesday, October 24, 2023
12:30 PM - 1:30 PM ET
.jpg)
Michael Zaleski
PhD Candidate
University of Pennsylvania
Philadelphia, Pennsylvania, United States.jpg)
Michael Zaleski
PhD Candidate
University of Pennsylvania
Philadelphia, Pennsylvania, United States- LC
Liam Chase
Research Specialist
University of Pennsylvania
Philadelphia, Pennsylvania, United States - JB
Jacob Brenner, M.D., Ph.D.
Associate Professor
University of Pennsylvania
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Drug delivery systems are typically constructed by chemically conjugating together multiple therapeutic molecules (e.g., antibody-drug conjugates/ADCs). Many conjugation chemistries, including the recent Nobel Prize-winning ‘click chemistry’, are used to produce these delivery systems. For ADCs, it is well-known that the choice of conjugation chemistry can have a profound impact on the efficacy and safety of the final product. But for newer delivery systems, such as protein-coated nanoparticles, the effect of conjugation chemistry is not well understood. Here we show that the choice of conjugation chemistry primarily affects the complement opsonization of nanoparticles. Complement activation subsequently has dramatic impacts on both safety (via release of anaphylatoxins) and efficacy (via increased clearance by phagocytes) of the final nanoparticle product.
Methods: Non-specific IgG was conjugated to the surface of liposomes using two of the most common conjugation chemistries: DBCO-azide and SATA-maleimide. Liposomes were radiolabeled with I-125 and injected into mice. Radioactivity in each organ was measured via gamma counter. IgG modified with SATA or DBCO were analyzed for aggregation via HPLC-SEC. Fluorescent molecules (AF488) and quenchers (TQ2) were conjugated to IgG via NHS-ester reaction. The IgG were then conjugated to the surface of liposomes via DBCO-azide or SATA-maleimide The fluorescence intensity of the liposomes was measured via a fluorometer, with decreased fluorescence meaning that some fluorescence was quenched, and therefore the IgG were located in close proximity on the liposome surface. Finally, bovine serum albumin (BSA) was fluorescently labeled with AF488 via NHS-ester reaction. Liposome with varying reactive groups (azide, maleimide, or non-reactive lipid) were incubated with AF488-albumin at 37C for 30 minutes. The fraction of liposomes attached to fluorescent albumin was analyzed via NTA (Nanoparticle Tracking Analysis)
Results: IgG-coated liposomes accumulated in the lung, likely due to uptake by marginated neutrophils. The extent of lung uptake varied for different conjugation chemistries (DBCO-azide and SATA-maleimide). Lung uptake was dramatically decreased in C3-knock out mice, and was the same across all conjugation chemistries. This suggests that conjugation chemistries vary in the extent they cause complement activation. Azide-alkyne chemistry (DBCO-azide) causes aggregation of protein on the nanoparticle surface, as measured by fluorescence quenching. This is likely due to the hydrophobic alkyne functional group. For thiol-maleimide chemistry, maleimide reacts non-specifically with albumin and other plasma proteins, leading to a dense protein-coated surface. In both cases, the dense protein structure and hydrophobic interactions increases the rate of complement opsonization.
Conclusion: The conjugation chemistry used to construct protein-coated nanoparticles dramatically effects the complement opsonization of the nanoparticle. The mechanism of complement activation is different for two of the most common conjugation chemistries. DBCO-azide causes protein aggregation on the surface of the liposome, which leads to complement opsonization. Maleimide functional groups on the liposome reacts non-specifically with proteins in plasma, especially albumin. This leads to a dense protein coating on the surface of the liposome, which increases complement opsonization. This new understanding of the effect of conjugation chemistry on complement activation will enable engineering of new nanoparticles with superior safety and efficacy.
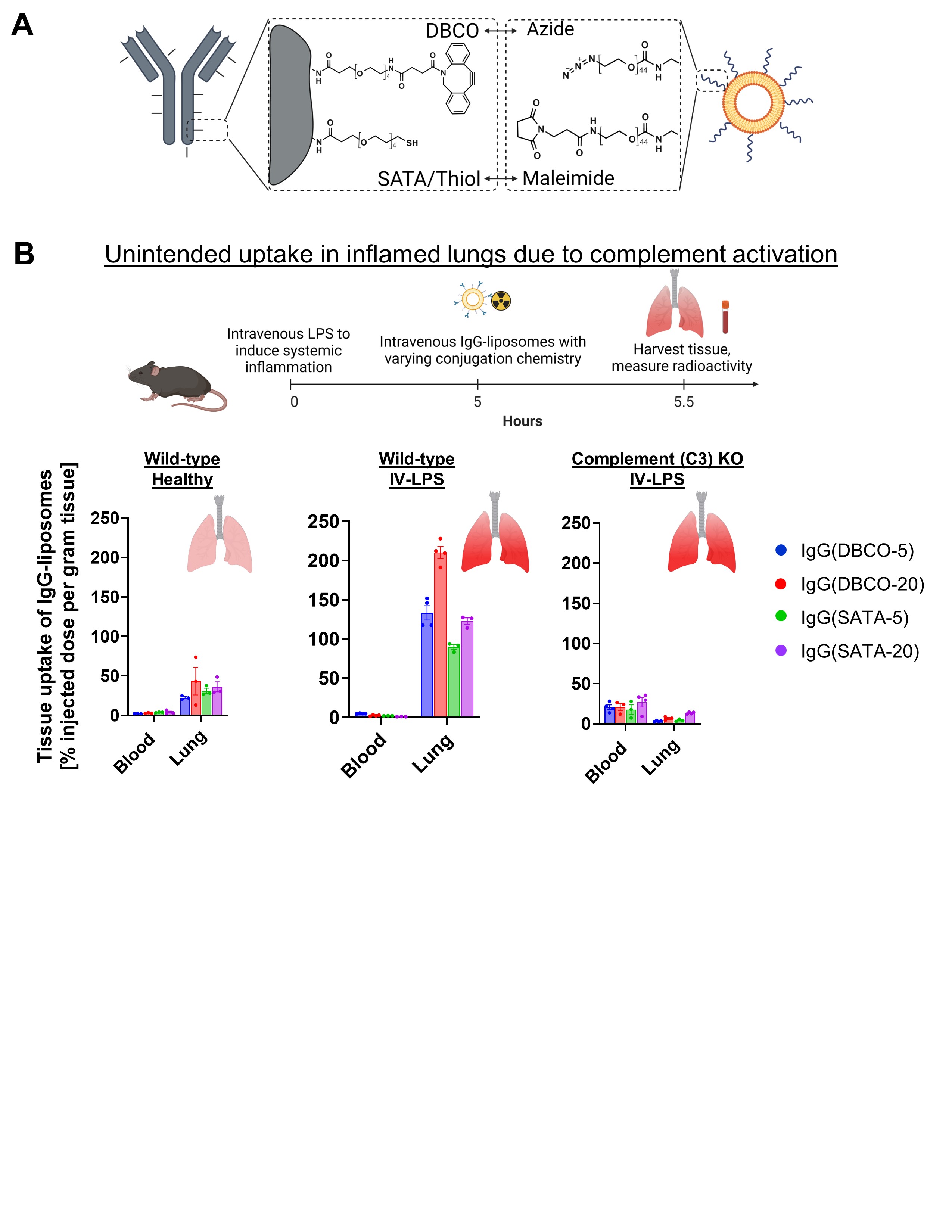 A) Schematic of two of the most common conjugation chemistries used to build drug delivery systems: DBCO-azide and SATA-maleimide. Note: A thiol is displayed in the schematic because SATA is deprotected to a thiol group for conjugation to maleimide.
A) Schematic of two of the most common conjugation chemistries used to build drug delivery systems: DBCO-azide and SATA-maleimide. Note: A thiol is displayed in the schematic because SATA is deprotected to a thiol group for conjugation to maleimide. B) Biodistribution of IgG-liposome conjugates in mice. Systemic inflammation was induced in mice via an intravenous injection of LPS (2 mg/kg). Five hours after LPS, mice were intravenously injected with radiolabeled liposomes (lipid dose of 3 mg/kg). Thirty minutes after liposomes injection, organs were collected and radioactivity was measured. Note that for healthy mice, there is low uptake in the lungs, and there is no difference between conjugation chemistries. In mice with systemic inflammation induced by IV-LPS, there is high uptake of liposomes in the lungs, and this is significantly affected by conjugation chemistry. Knocking out complement protein C3 completely prevents lung uptake of antibody-conjugated liposomes.
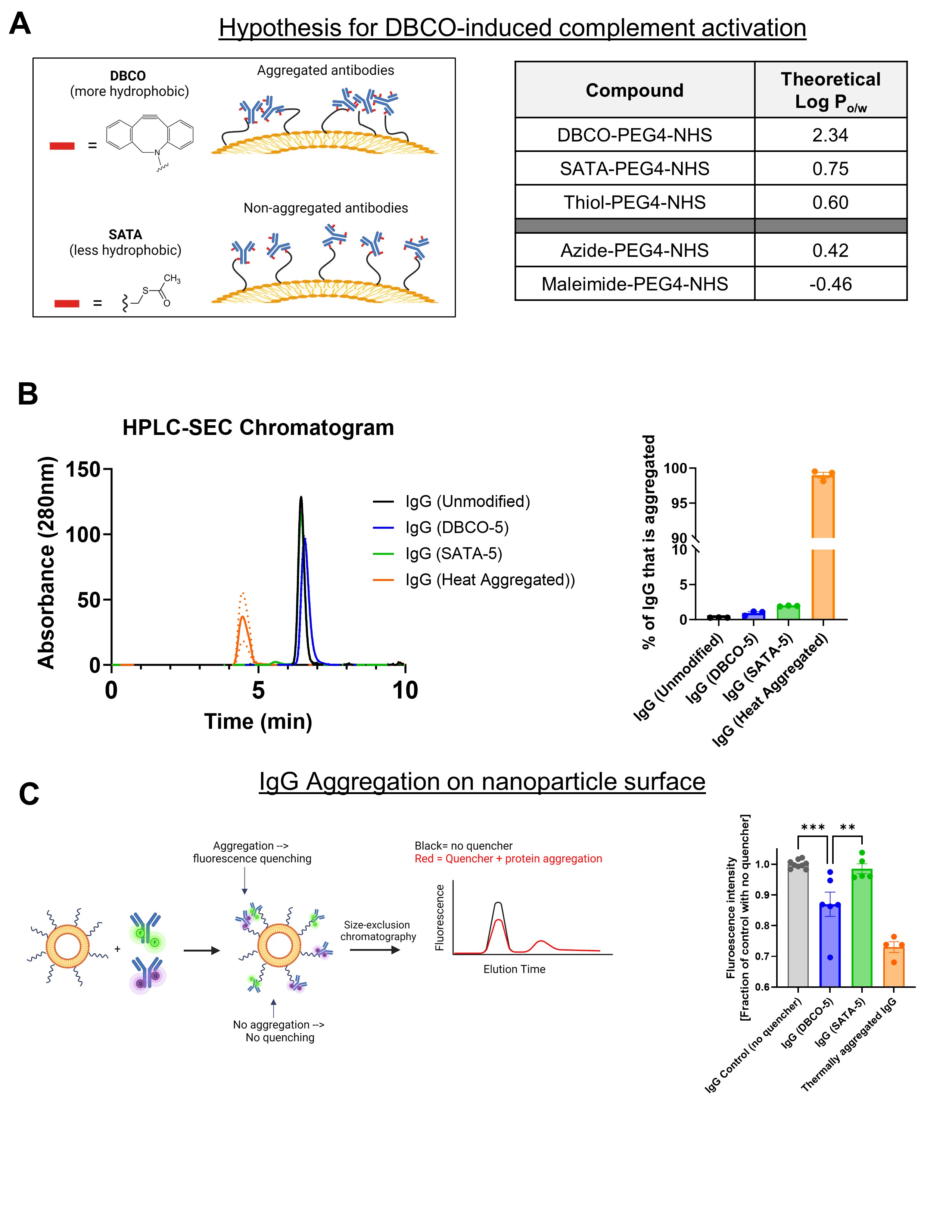 A) Schematic of hypothesis for mechanism of DBCO influence on complement activation. Due to the hydrophobicity of the DBCO moiety, proteins aggregate on the nanoparticle surface. Theoretical hydrophobicity of common moieties used in bioconjugation. Theoretical Log(P) was calculated using Swiss ADME online tool. DBCO is two orders-of-magnitude more hydrophobic than SATA and thiol (after SATA deprotection) moieties.
A) Schematic of hypothesis for mechanism of DBCO influence on complement activation. Due to the hydrophobicity of the DBCO moiety, proteins aggregate on the nanoparticle surface. Theoretical hydrophobicity of common moieties used in bioconjugation. Theoretical Log(P) was calculated using Swiss ADME online tool. DBCO is two orders-of-magnitude more hydrophobic than SATA and thiol (after SATA deprotection) moieties. B) Size-exclusion HPLC analysis of modified IgG. Representative chromatograms show the retention time, with larger molecular weight molecules (indicating aggregated protein) eluting earlier.
C) Schematic for and data fluorescent quenching analysis. IgG are modified with either a fluorescent molecule or a dark quencher. After conjugation of IgG to the liposome, the total fluorescence is decreased if fluorescent and quencher molecules are in close proximity (e.g., due to IgG aggregation). Heat-induced aggregation is used as a positive control.
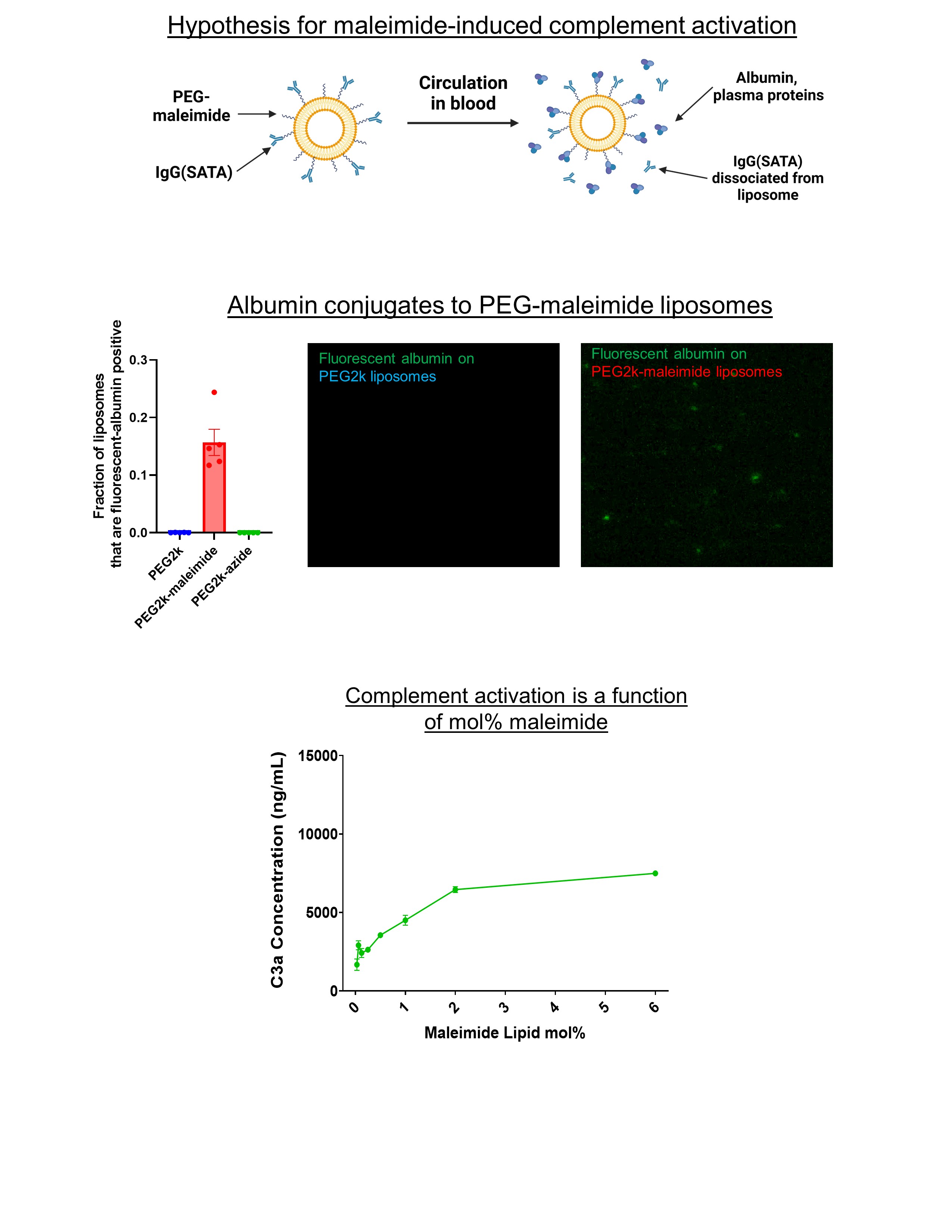 A) Schematic of hypothesis for mechanism of SATA-maleimide influence on complement activation. Maleimide reacts non-specifically with proteins in plasma, particularly albumin. This leads to a dense protein coating on the surface of the liposome, which initiates complement opsonization.
A) Schematic of hypothesis for mechanism of SATA-maleimide influence on complement activation. Maleimide reacts non-specifically with proteins in plasma, particularly albumin. This leads to a dense protein coating on the surface of the liposome, which initiates complement opsonization. B) Florescent analysis of albumin binding to liposomes. Bovine serum albumin (BSA) was modified with a fluorescent tracer and incubated with liposomes containing various reactive lipids. Fluorescence of liposomes was measured via NTA (Nanoparticle Tracking Analysis) and showed that albumin bound to maleimide liposomes, but not azide liposomes.
C) Liposomes with varying amount of maleimide reactive lipids were incubated with serum, and complement activation was measured via ELISA of C3a (a protein produced upon complement activation).
