Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(T1130-12-82) Quality by Design (QbD) Based Formulation and Process Optimization for a Direct Blend Process: A Risk Based Industrial Approach
Tuesday, October 24, 2023
11:30 AM - 12:30 PM ET
- RS
Robert Sciscento, M.S.
Alcami Corporation
wilmington, North Carolina, United States - SG
Saujanya Gosangari, Ph.D.
Alcami Corporation
wilmington, North Carolina, United States
Presenting Author(s)
Main Author(s)
Purpose: Direct blend processes for solid dose products are subjected to several challenges such as segregation due to differences in density, particle size distribution profile of the APIs and excipients used. This segregation in turn can impact downstream operations such as compression/encapsulation and result in weight variation and content uniformity issues. Identification and control of critical material attributes, formulation variables such as component ratios, critical process parameters and a thorough understanding of their interplay are key aspects of product development and characterization. The objective of this study was to use a quality by design approach in order to develop a comprehensive understanding of the above stated for a direct blend formulation and process. Extensive characterization of the powder blend was performed to understand the impact of the different formulation and process variables on final blend characteristics and encapsulation feasibility.
Methods: An experimental design (DOE) was created which incorporated two levels for four variables evaluated in the DOE. Eighteen batches consisting each of 400 gram batch size was performed. Effects of different particle size distribution for colloidal silicon dioxide and magnesium stearate were not studied as each of these excipients constitute less than 1%w/w in the formulation. Two lots of API with different particle size distributions were supplied and used “as received”. Microcrystalline Cellulose (MCC) was supplied by FMC Biopolymer™ and used as received or sieve cut to obtain fine and coarse material. The particle size distribution data of API lots and microcrystalline cellulose lots were obtained using sieve analysis and the geometric mean, D10, D50, D90 values were generated. From a process standpoint, main blend revolutions and lubricant blend revolutions were challenged as part of the study design. Minitab statistical software was used to design the study and randomize the experimental order. In order to increase the understanding of the interaction of early formulation variables interactions and their influence on the later production process, a risk assessment followed by a design of experiments approach was conducted.
Results: The flowability of 18 DOE runs are compared in Figure 1. All the blends flow through an orifice of 4mm. Therefore, there was no difference in flow properties of the 18 DOE runs, suggesting that the particle size of the Microcrystalline Cellulose (MCC) and API, and different blending times did not have influence on the flow of the final blend. The dissolution profiles graphs of the 18 runs are displayed in Figure 2. From the figure, visually no difference in the release profile of all the 18 runs can be seen. The capsules from all the 18 runs showed ~ 100% release at the end of 10 minutes. Therefore, 10 minutes dissolution data will be used for statistical analysis. Figure 3 compares the Main effects on BU-Mean, CU-Mean, % Dissolution Mean. The API PSD had an inverse relationship on all the three responses, following a trend. Therefore, caution must be taken to avoid using coarse particle size of the API. The MCC PSD and Main blend time had negligible effect on all the three responses. In case of lubrication blend time, the effect did not follow a pattern, but the magnitude of the effect was high. Therefore, caution should be taken to avoid under or over blending the batch after addition of the lubricant.
Conclusion: The process of blending, milling, and encapsulation for all the 18 batches proceeded smoothly. The physical properties like bulk/tap density using tap density apparatus, flowability by Flodex, and particle size analysis using sonic sifter on all 18 batches was successfully performed. There was no substantial difference in bulk/tap density and flow between the 18 batches. There were differences seen in the particle size of the 18 batches as designed by the experiment. To summarize, the particle size of 18 batches of blends can be divided into two groups. One group that had coarse Microcrystalline Cellulose (MCC, Avicel PH102) showed greater percentage of particles retained on 80,100, and 140 mesh sieve. The second group with fine and intermediate Microcrystalline Cellulose (MCC, Avicel PH102) had greater percentage of particles retained on 200 and 325 mesh sieve and collection pan. All 18 runs passed the acceptance criteria for blend uniformity, content uniformity, and dissolution. Differences in the results were statistically insignificant. None of the main variables or their interactions affected the responses which demonstrate a robust formulation and process. However, maximum effects were seen in case of lubrication blending time. Therefore, in manufacturing large scale batches, care must be taken to avoid extreme conditions of lubrication blend time that may increase the variability of the analytical quality test results.
.jpg) Figure 1. Flowability Comparisons of 18 QbD Runs
Figure 1. Flowability Comparisons of 18 QbD Runs 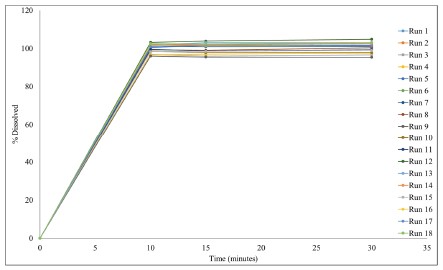 Figure 2. Dissolution Profile Comparisons for the 18 DOE runs
Figure 2. Dissolution Profile Comparisons for the 18 DOE runs 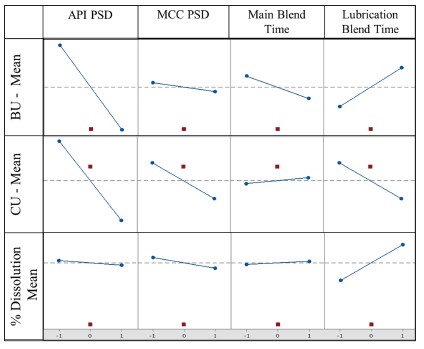 Figure 3. Comparison of Main effects of BU-Mean, CU-Mean and % Dissolution Mean
Figure 3. Comparison of Main effects of BU-Mean, CU-Mean and % Dissolution Mean