Formulation and Delivery - Biomolecular
Category: Late Breaking Poster Abstract
(T1130-12-77) Vitamin D and Insulin Microparticle Incorporation into 3D-Printed Gene-Activated Calcium Phosphate Cement Scaffolds for Bone Regeneration Applications
Tuesday, October 24, 2023
11:30 AM - 12:30 PM ET

Esraa Mohamed, PhD (she/her/hers)
PhD Graduate Student
University of Iowa
Iowa City, Iowa, United States- NL
Noah Laird (he/him/his)
University of Iowa
Iowa city, Iowa, United States - PP
Pornpoj Phruttiwanichakun (they/them/theirs)
University of Iowa
Iowa City, Iowa, United States - DF
Douglas Fredericks (they/them/theirs)
University of Iowa
Coralville, Iowa, United States - JF
John M. Femino (they/them/theirs)
University of Iowa
Iowa City, Iowa, United States - AS
Aliasger Salem, Ph.D.
University of Iowa
Iowa City, Iowa, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The goal of this study was to evaluate the impact on bone regeneration and osseointegration of combined local delivery of insulin (INS) and vitamin D (VitD3) with nonviral gene delivery of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2). Bone tissue engineering holds great promise for regenerating bone in large defects, thereby eliminating the need for grafts or prosthetics. Such therapies have the potential to significantly improve both long- and short-term outcomes for patients with critical-sized bone defects. One approach that shows considerable potential is the use of gene-activated scaffolds designed to deliver nucleic acids which can promote local tissue repair. In addition, INS and VitD3 have emerged as key factors in inducing bone turnover and stimulating osteogenesis. While there is evidence suggesting that INS can enhance osteogenesis, its application in bone regeneration has received relatively little attention, possibly due to the short half-life of INS and the potential of side effects of excessive INS at defect site. Therefore, the development of a controlled, localized INS delivery system become imperative to maintain bioactivity and appropriate drug concentration at the site of bone, thereby facilitating effective bone regeneration.
Methods: We developed a novel composite biomaterial capable of simultaneously delivering INS, VitD3, BMP-2, and FGF-2. The bioactivity of the composite scaffolds was thoroughly examined in vitro and in vivo using a rat critical size defect model. The composite consisted of a 3D-printed calcium phosphate cement (CPC) scaffold loaded with polyethyleneimine (PEI)-(BMP-2 and FGF-2) nanoplexes and vitD3 (inner layer). Additionally, an outer layer of collagen ribbon containing INS-loaded PLGA microparticles was incorporated. We optimized a mineralization method and post-mineralization treatment using either water, vapor and/or simulated body fluid (SBF) to enhance cell adhesion to the scaffolds and facilitate the regenerative processes which involve scaffold resorption and new bone formation. We evaluated the transfection efficiency in vitro of the (PEI)-(BMP-2 and FGF-2) nanoplexes over time. We prepared the INS-loaded MPs using a double emulsion method and the particles were characterized in terms of mean particle size, drug loading, entrapment efficiency and surface morphology. Using a rat model with long bone defects, we implanted the scaffold, loaded with PEI-(BMP-2 and/or FGF-2) nanoplexes and VitD3 and wrapped with the collagen ribbon containing the INS-loaded MPs, at the defect site. The animal study is currently ongoing to assess the outcomes and results.
Results: The gene activated 3D-printed CPC scaffold treated with vapor and SBF showed surface roughness sufficient for cell adhesion and higher transfection efficiency. The INS-loaded microparticles exhibited a mean particle size of a 23.74 ± 5.5 µm with an INS loading of 51.16± 0.47 µg INS/mg MPs and an entrapment efficiency of 79 ± 9.89%. The scanning electron microscopy (SEM) images of the empty and insulin loaded MPs showed spherical particles with a smooth surface. Additionally, our in vitro release study demonstrated that the scaffold was able to achieve a sustained release of VitD3.
Conclusion: The data shown here demonstrate that our composite scaffolds was able to adhere and transfect cells having the potential to significantly improve both long- and short-term outcomes for patients with critical-sized bone defects. Additionally, the composite was able to release VitD3 in a sustained manner. The incorporation of INS into PLGA microparticle has the potential to enhance the role of INS in bone regeneration by releasing INS over time. We are still waiting for the animal study results to confirm our hypothesis that the addition of INS and VitD3 to the gene-activated scaffold can enhance bone regeneration.
Acknowledgements: Esraa Mohamed would like to acknowledge the support of the Egyptian Government Ministry of Higher Education Missions Sector sponsorship. The authors would like to acknowledge use of the University of Iowa Central Microscopy Research Facility, a core resource supported by the University of Iowa Vice President for Research, and the Carver College of Medicine. I would like to acknowledge the help and guidance received from Noah Z. Laird, Pornpoj “Jay” Phruttiwanichakun, Dr. Aliasger Salem, Dr. Sean Geary, and other members of the Salem Lab who have helped with this project.
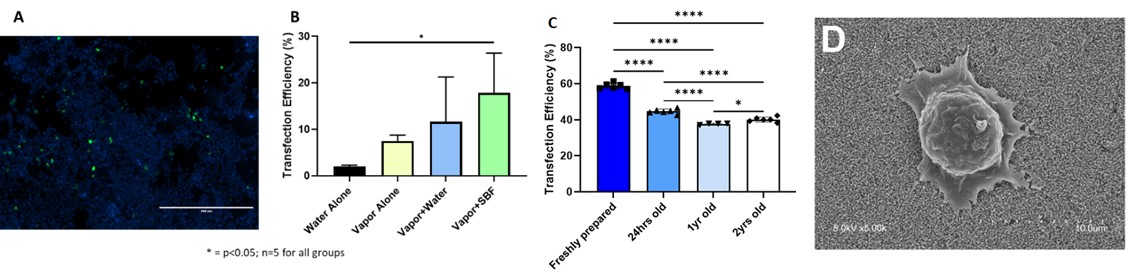 Figure 1: Fluorescence micrograph (A) Transfection efficiency of HEK 293T cells seeded onto gene-activated CPC discs as determined by flow cytometry; Bars represent mean + SD, n=5 for all groups for different treatment methods (B) transfection efficiency of nanoplexes confirming the long term stability of nanoplexes to deliver the pDNA (C) and Micrographs of HEK 293T cells incubated for 4 hours on printed scaffolds mineralized with vapor then simulated body fluid (D).
Figure 1: Fluorescence micrograph (A) Transfection efficiency of HEK 293T cells seeded onto gene-activated CPC discs as determined by flow cytometry; Bars represent mean + SD, n=5 for all groups for different treatment methods (B) transfection efficiency of nanoplexes confirming the long term stability of nanoplexes to deliver the pDNA (C) and Micrographs of HEK 293T cells incubated for 4 hours on printed scaffolds mineralized with vapor then simulated body fluid (D). .jpg) Figure 2: SEM images of (A) Blank MPs and (B) Insulin loaded MPs and (C) Cumulative release of calcitriol (vitamin D) from CPC scaffolds (Values represent mean + SD, n=6 for all samples).
Figure 2: SEM images of (A) Blank MPs and (B) Insulin loaded MPs and (C) Cumulative release of calcitriol (vitamin D) from CPC scaffolds (Values represent mean + SD, n=6 for all samples)..jpg) Figure 3: (A) CPC scaffold next to collagen ribbon that contains insulin-loaded microparticles, (B) CPC scaffold wrapped in collagen ribbon, (C) CPC scaffold/collagen ribbon during implantation in the defect site (D) X-ray of CPC scaffold/collagen ribbon after implantation.
Figure 3: (A) CPC scaffold next to collagen ribbon that contains insulin-loaded microparticles, (B) CPC scaffold wrapped in collagen ribbon, (C) CPC scaffold/collagen ribbon during implantation in the defect site (D) X-ray of CPC scaffold/collagen ribbon after implantation.