Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(M1530-03-18) Understanding the Role of Particle Size of Poloxamer P407 on Properties of Tablet Prepared by Direct Compression Process
Monday, October 23, 2023
3:30 PM - 4:30 PM ET
- MJ
Ming Ji, Ph.D.
BASF Corporation
Tarrytown, New York, United States - MJ
Ming Ji, Ph.D.
BASF Corporation
Tarrytown, New York, United States - NS
Nitin Kumar Swarnakar, Ph.D.
BASF Corporation
Tarrytown, New York, United States - ST
Sandip Tiwari, Ph.D.
BASF Corporation
Tarrytown, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Poloxamer P407 has been used as a surfactant increase the solubility of poorly soluble active pharmacal ingredients (API) because of the micelle formation property in aqueous environment at certain concentrations. Additionally, it could be considered as a lubricant for direct tablet compression (1). This study aims to investigate the effect of particle size of Poloxamer P407 on tablet properties and dissolution of a model drug (ibuprofen), using the new an all-in-one co-processed direct compression excipient (KollitabTM DC 87L).
Methods: Ibuprofen (IBU) 70, poloxamer P407 (Kolliphor® P407) micro and the co-processed excipient (KollitabTM DC 87L) were passed through a #40 mesh sieve to de-lump agglomerated materials before mixing. Regular grade P407 (mean particle size ~500 µm) was passed through a #20 mesh sieve. The excipients and Ibuprofen 70 were passed through the sieves before mixing by two ways: 1) each individual excipient and Ibuprofen 70 was passed the sieve represented as a non-co-sieved process; 2) all excipients and Ibuprofen 70 preblended were pass through the sieves represented as a co-sieved process. After sieving, the mixture of IBU 70 with P407 (regular/micro) and the co-processed excipient (15, 5, and 80 % w/w, respectively) was blended using V-shape blender at 25 rpm for 10 minutes. The flow properties of the blend were determined by Hausner Ratio (HR) and Carr’s Index (CI) (2). Tablet of 300 mg with a diameter of 9 mm containing 15 mg ibuprofen were compressed by using a Korsch XL100 Pro Tablet Press. The dissolution was tested using USP Type II apparatus (Pion MacroFLUXTM with Erweka GmbH) with 0.1N HCl solution maintained at 37 ± 0.5°C and stirred at 75 rpm.
Results: IBU 70 blend with 5% poloxamer P407 and 80% the co-processed excipient shows good/passable flowability based on the Hausner ratio and Carr's index (Table 1). Because of the larger particle size of regular poloxamer P407 (mean particle size ~500 µm), IBU/regular P407 blend has better flowability compared to the blend with P407 micro (mean particle size ~50 µm). The better flowability in case of the blend containing P407 with higher particle size reflects easier tablet manufacturing but demonstrated tablet sticking and segregation problems during the compression process. This suggested that P407 regular grade needs to be milled to smaller particle size in order to avoid segregation problem. However, it is difficult to do the milling process at room temperature because of the low congealing temperature of P407 (~53°C). In contrast, P407 micro blend doesn’t require the milling procedure and still have provided comparable flowability without segregation (Table 1). During continuous compression, tablet picking occurred when simple blending (non-co-processed sieving) was used . However, no picking happened during the continuous tablet manufacturing when co-processed sieving procedure was incorporated. No change in the thermogram upon differential scanning calorimetry (DSC) testing confirmed no chemical or eutectic mixture forming between IBU-poloxamer in blends prepared by non co-sieving and co-sieving methods. Therefore, uniform distribution of P407 micro particle in IBU-the co-processed excipient blend was achieved by co-sieve of all excipients followed by mixing to avoid tablet picking issue. To check the solubilization effect of P407 (regular vs micro), dissolution of the IBU tablets were tested with/without poloxamer P407 in 0.1N HCl. The saturation concentration of IBU in 0.1N HCl was found to be ~63 mg/mL after 6 hours. The presence of 5% P407 regular or micro in the formulation significantly improved the solubilization of API (~84 mg/mL and ~80mg/mL, respectively). This study proves that the existence of poloxamer P407 in the IBU tablet increase the solubility of IBU in 0.1N HCl by ~30%.
Conclusion: IBU blend with poloxamer P407 micro demonstrated better tablet manufacturing (no segregation and sticking problem) than P407 regular with slight comprised flow properties as compared to the blend with P407 regular. Additionally, size reduction of regular grade P407 is challenging using milling at room temperature because of its low congealing temperature (~53°C). In contrast, P407 micro did not require any milling procedure and have provided the similar solubility enhancement to P407 regular. In summary, P407 micro should be a better choice for continuous manufacturing of tablet containing poorly soluble API as compared to P407 regular.
References: [1] J. Muzikova, B. Vyhlidalova, T. Pekarek, Acta Poloniae Pharmaceutica – Drug Research, 2013, 70: 1087-1096.
[2] C. Turchiuli, M. Fuchs, M. Bohin, M.E. Cuvelier, C. Ordonnaud, M.N. Peyrat-Maillard, E. Dumoulin, Innovative Food Sci. Emerg. Technol. 2005, 6: 29–35.
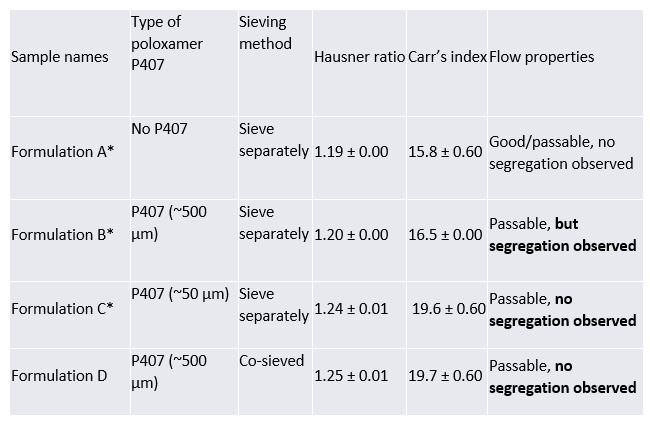 Tapped density testing results of IBU/poloxamer P407/the co-processed excipient blend. Formulation A: 15% Ibuprofen 70 + 85% the co-processed excipient ; Formulation B: 15% Ibuprofen 70 + 5% poloxamer P407 (regular) + 80% the co-processed excipient ; Formulation C: 15% Ibuprofen 70 + 5% poloxamer P407 (micro) + 80% the co-processed excipient ; Formulation D: 15% Ibuprofen 70 + 5% poloxamer P407 (micro) + 80% the co-processed excipient, co-processed sieving.
Tapped density testing results of IBU/poloxamer P407/the co-processed excipient blend. Formulation A: 15% Ibuprofen 70 + 85% the co-processed excipient ; Formulation B: 15% Ibuprofen 70 + 5% poloxamer P407 (regular) + 80% the co-processed excipient ; Formulation C: 15% Ibuprofen 70 + 5% poloxamer P407 (micro) + 80% the co-processed excipient ; Formulation D: 15% Ibuprofen 70 + 5% poloxamer P407 (micro) + 80% the co-processed excipient, co-processed sieving..jpg) Dissolution profile of IBU tablets in 0.1N HCl media maintaining at 37°C and stirring at 75 rpm, each sample tested three times.
Dissolution profile of IBU tablets in 0.1N HCl media maintaining at 37°C and stirring at 75 rpm, each sample tested three times.