Preclinical and Translational Sciences - Biomolecular
Category: Late Breaking Poster Abstract
(M1330-10-65) Quantifying Clinical Trial Diversity of New Drugs Approved by the FDA
Monday, October 23, 2023
1:30 PM - 2:30 PM ET

Kay Olmstead, PhD (she/her/hers)
CEO
Nano PharmaSolutions
San Diego, California, United States- WF
William Fitzsimmons, Pharm.D.
University of Illinois Chicago
Chicago, Illinois, United States - SL
Stanley Lewis, M.D.
A28 Therapeutics
La Jolla, California, United States - EF
Elizabeth Fassberg, M.S. (she/her/hers)
Life Science Cares Network
New York, New York, United States - CK
Chris Komelasky, M.A. (he/him/his)
SiteBridge Research
Chapel Hill, North Carolina, United States - MI
Muhammed Idris, Ph.D. (he/him/his)
Morehouse School of Medicine
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Diversity representation in pivotal Phase 3 registration trials for new drugs is critical to ensuring safety and efficacy in under- served segments of the population. Underrepresentation by any specific population group not only has economic implications, but more importantly, it perpetuates health inequality. We introduce a Clinical Trial Diversity (CTD) Scorecard, an instrument designed to evaluate diversity in the registrational clinical trial for new molecular entities and new therapeutic biological products approved by US FDA.
Methods: FDA review documents for all novel drug approvals from January 2022 through February 16, 2023 were assessed using a scorecard that considers diversity across different demographic subgroups including age ( >65 yo) , sex (female) , race (Black and Asian) and ethnicity (Hispanic/Latino). The scorecard assigns each drug a letter grade, between A and F, for each subgroup (and overall) based on 1) the percent of each sub-population included in the trials and grades relative to the percent of the US population, 2) the number of participants from each subpopulation that received the novel new drug in the trials, 3) the incidence or prevalence of the disease/condition in each of the sub-populations.
Results: The FDA approved 43 novel new drugs for 44 indications (one drug was simultaneously approved for two indications). The three drugs with A Grades reflecting the best diversity in their registration trials were tapinarof (Vtama from Dermavant), daprodustat (Jesduvroq from GlaxoSmithKline) and eflapegrastim (Rolvedon from Spectrum Pharmaceuticals.) There was good representation of elderly and females with only two drugs receiving a D grade in either of these sub-populations. In contrast, Black and Hispanic representation was often inadequate with 4 drugs receiving F grades for the specific subgroup participation. There were 9 drugs (20%) where there were no Black participants receiving the novel new drug and an additional 14 approvals where there were < 10 Black participants receiving the novel drug. The median number of Black participants receiving the investigational drug was 9 for these 43 drugs. In the Hispanic/Latino population there were 2 approvals with no Hispanic participants receiving the novel drug and 14 approvals where there were < 10 Hispanic participants receiving the drug. The median number of Hispanic participants receiving the novel drug was 12.5.
Conclusion: This newly developed scorecard provides an objective quantitative approach to assess the current state of diversity in clinical trials supporting new drug approvals. Substantial improvement in racial and ethnic representation is needed. Meaningful change will require actions and cooperation amongst all stakeholders to address this multifaceted issue and will take commitment, perseverance, and appropriate incentives.
References: 1. National Academies of Sciences, Engineering, and Medicine 2022. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington, DC: The National Academies Press. https://doi.org/10.17226/26479.
2. GAO-23-105245 CANCER CLINICAL TRIALS; Federal Actions and Selected Non-Federal Practices to Facilitate Diversity of Patients. December, 2022.
3. DEPARTMENT OF HEALTH AND HUMAN SERVICES; Food and Drug Administration; [Docket No. FDA–2021–D–0789] Diversity Plans To Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability AGENCY: Food and Drug Administration, HHS. ACTION: Notice of availability. Federal Register / Vol. 87, No. 72 / Thursday, April 14, 2022 / Notices.
4. H.R.2617 - Consolidated Appropriations Act, 2023 https://www.congress.gov/bill/117th-congress/house-bill/2617.
5. New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products https://www.fda.gov/drugs/development-approval-process-drugs/new-drugsfda-cders-new-molecular-entities-and-new-therapeutic-biological-products.
6. Drugs@FDA: FDA-Approved Drugs https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
7. United States Census Bureau QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045221
8. European Commission. A GUIDELINE ON SUMMARY OF PRODUCT
CHARACTERISTICS (SmPC) September 2009. https://health.ec.europa.eu/system/files/2016-11/smpc_guideline_rev2_en_0.pdf.
9. Burns, J. (2015). If Nothing Goes Wrong, Is Everything All Right? Why We Should Be Wary of Zero Numerators*. Pediatric Critical Care Medicine, 16 (2), 198-199. doi:10.1097/PCC.0000000000000346.
10. Lolic M, Araojo R, Okeke M, et al. Racial and Ethnic Representation in US Clinical Trials of New Drugs and Biologics, 2015-2019. JAMA. 2021 Dec 7;326(21):2201-2203. doi: 10.1001/jama.2021.16680. PMID: 34874429; PMCID: PMC8652601.
11. Varma T, Miller JE. Ranking pharmaceutical companies on clinical trial diversity. BMJ. 2023 Feb 10;380:334. doi: 10.1136/bmj.p334. PMID: 36764687.
12. Reid MM, Davis SP, Henry ON, et. al. Demographic diversity of US-based participants in GSK-sponsored interventional clinical trials. Clin Trials. 2023. Apr;20(2):133-144. doi: 10.1177/17407745221149118. Epub 2023 Feb 6. PMID:
36744680; PMCID: PMC10021118.
13. Peters U, Turner B, Alvarez D, et. al. Considerations for Embedding Inclusive Research Principles in the Design and Execution of Clinical Trials. Ther Innov Regul Sci. 2023 Mar;57(2):186-195. doi: 10.1007/s43441-022-00464-3. Epub 2022 Oct 14. PMID: 36241965; PMCID: PMC9568895.
14. Varma T, Mello M, Ross JS, et. al. Metrics, baseline scores, and a tool to improve sponsor performance on clinical trial diversity: retrospective cross sectional study. BMJ Med. 2023 Jan 4;2(1):e000395. doi: 10.1136/bmjmed-2022-000395. PMID: 36936269; PMCID: PMC9951369.
15. Blumenthal D, James CV. A Data Infrastructure for Clinical Trial Diversity. N Engl J Med. 2022 Jun 23;386(25):2355-2356. doi: 10.1056/NEJMp2201433. Epub 2022 Apr 27. PMID: 35476634.
16. Varma T, Jones CP, Oladele C, et. al.. Diversity in clinical research: public health and social justice imperatives. J Med Ethics. 2022 Apr 15:medethics-2021-108068. doi: 10.1136/medethics-2021-108068. Epub ahead of print. PMID: 35428737
17. Schwartz AL, Alsan M, Morris AA, et al. Why Diverse Clinical Trial Participation Matters. N Engl J Med. 2023 Apr 6;388(14):1252-1254. doi: 10.1056/NEJMp2215609. Epub 2023 Apr 1. PMID: 37017480.
.jpg) Clinical Trial Diversity Scores of New Drugs Approved in 2022 by the FDA_Page 1
Clinical Trial Diversity Scores of New Drugs Approved in 2022 by the FDA_Page 1.jpg) Clinical Trial Diversity Scores of New Drugs Approved in 2022 by the FDA_Page2
Clinical Trial Diversity Scores of New Drugs Approved in 2022 by the FDA_Page2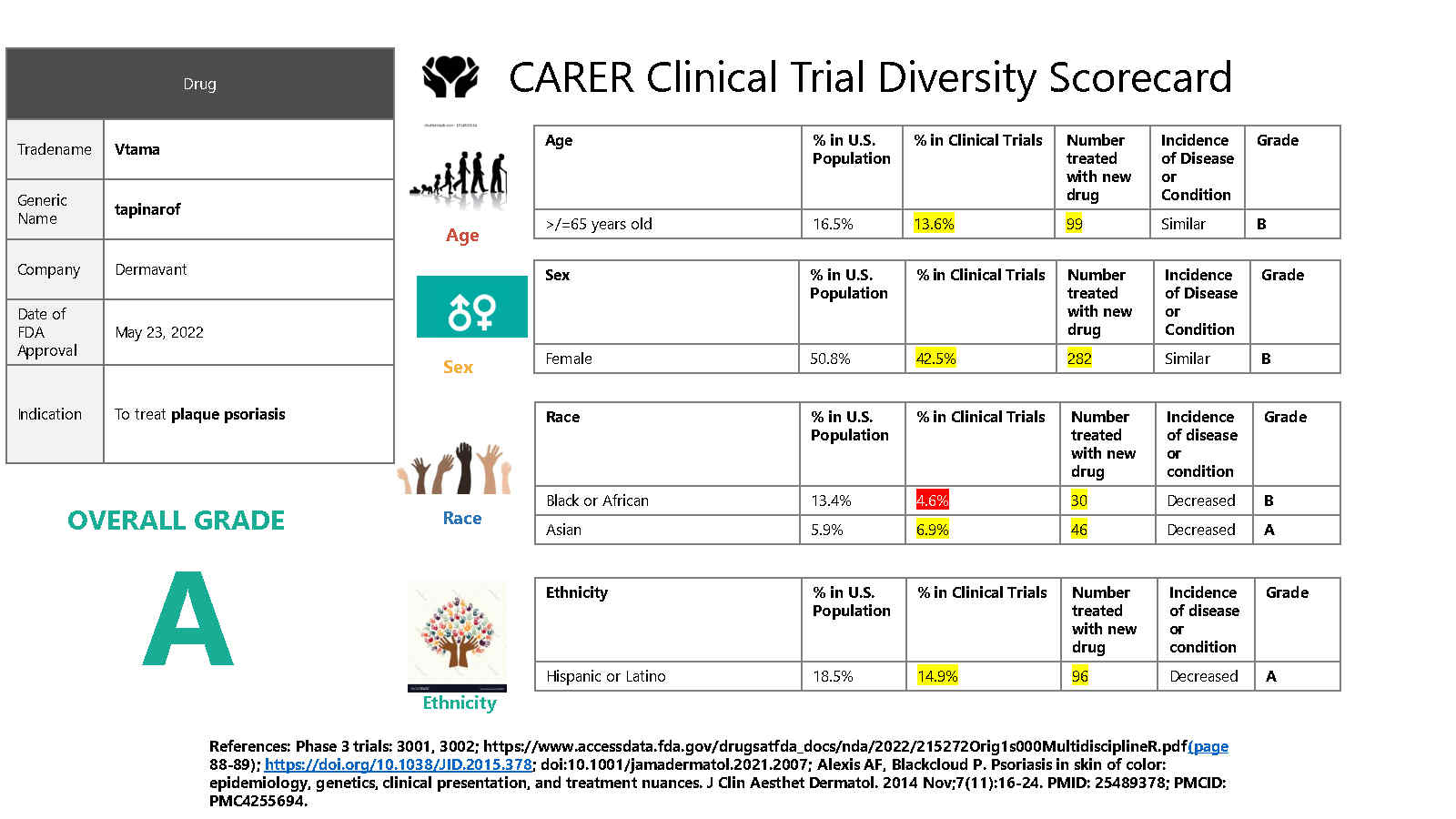 Clinical Trial Diversity Score Card of Year 2022- Highest Grade
Clinical Trial Diversity Score Card of Year 2022- Highest Grade