Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(M1230-12-79) Characterization and Performance of 60% Drug Loaded Freeze-Dried Amorphous Nanoparticle Tablet of a BCS IV Compound: ABT-530
Monday, October 23, 2023
12:30 PM - 1:30 PM ET

Nasim Ganji, PhD (she/her/hers)
Postdoctoral Scientist
AbbVie Inc.
North Chicago, Illinois, United States
Nasim Ganji, PhD (she/her/hers)
Postdoctoral Scientist
AbbVie Inc.
North Chicago, Illinois, United States- HO
Hardeep Oberoi, Ph.D.
AbbVie Inc.
North Chicago, Illinois, United States - MY
Mengqi Yu, Ph.D.
AbbVie Inc.
North Chicago, Illinois, United States - DL
Devalina Law, Ph.D. (she/her/hers)
AbbVie Inc.
North Chicago, Illinois, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Amorphous solid dispersion (ASD) formulations are widely used to enhance the solubility of poorly water-soluble compounds. However, the current ASD formulations have limitations in terms of drug loading (DL), typically ≤ 25% (w/v). This results in a high pill burden for patients, as larger tablet sizes are required to deliver the desired dose. In a previous study, we developed amorphous nanoparticle intermediates with high DL (≥ 60% (w/v)) using spray drying and lyophilization techniques. Although these intermediates demonstrated excellent re-dispersibility, they still required further processing to convert them into tablet/capsule formulations. The objective of this research is to develop a high drug load amorphous nanoparticle final dosage form, specifically tablets capable of releasing drug-rich nanoparticles upon contact with an aqueous environment.
Methods: We utilized a BCS IV compound, ABT-530 as a model and employed a controlled solvent-antisolvent precipitation method to generate a dilute suspension containing drug-rich amorphous nanoparticles (ANPs) with a high drug content of 90% (w/w). The precipitation process was optimized to yield a suspension with ANPs < 200 nm. Subsequently, the nanoparticle suspension was concentrated using tangential flow filtration (TFF), and the polymer PVPVA was added to enable direct lyophilization of the suspension in preformed tablet blisters. The impact of process variables on the formulation intermediate and the final dosage form was investigated using various characterization techniques including dynamic light scattering (DLS), scanning electron microscopy (SEM), high-performance liquid chromatography (HPLC), texture analyzer (TX), micro computed tomography (µ-CT) scanning, and Brunauer-Emmett-Teller (BET) surface area measurement.
Results: The resulting freeze-dried tablet had DL of 60% (w/v) and solid loading ranging from 24% (w/v) to 32% (w/v). The tablets maintained their structural integrity, as indicated by an intact cake occupying the same volume as the original frozen form with no signs of collapse or shrinkage. The ABT-530 Tablets prepared using this approach exhibited an elegant appearance and demonstrated sufficient mechanical strength to endure handling and transportation. Notably, as shown in Figure 1A, the 1.25% (w/v) solid containing ANP suspension post IJ, the 41.45% (w/v) solid containing suspension post TFF, the suspension obtained from re-dispersing 24% (w/v) & 32% (w/v) solid containing tablets had comparable primary nanoparticles, ensuring efficient drug release from the tablets. HPLC analysis showed that re-dispersibility of the tablets decreases as the solid loading in the pre-lyophilization suspension increases from 24% (w/v) to 32% (w/v) (Figure 1B). When the solid loading in the suspension was 24% (w/v), more than 80% of the particles derived from the tablets were smaller than 0.45 mm after re-dispersion. However, when the solid loading was increased to 32% (w/v), the percentage of particles smaller than 0.45 µm decreased to about 70%. CD spectroscopy and SEM imaging revealed that ABT-530 Tablets derived from a 24% (w/v) solid load suspension exhibited higher porosity (73.13%) compared to those from a 32% (w/v) solid load suspension (65.26%) porosity. Additionally, the tablets with lower solid loading showed slightly larger pore sizes. SEM analysis confirmed the presence of nanoparticles embedded within the porous matrix of the tablets (Figure 2). The specific surface area of the ABT-530 Tablets was measured to be 0.4226 ± 0.0036 m2/g for tablets prepared from a 24% (w/v) solid load suspension and 0.2943 ± 0.0020 m2/g for tablets prepared from a 32% (w/v) solid load suspension.
Conclusion: Considering that current ASD formulation of Pibrentasvir (ABT-530) tablets achieve a DL of 10% (w/v), our approach yielding 60% (w/v) DL represents a significant improvement in the development of high drug load final dosage forms (Figure 3). The ability to deliver a higher drug content within a smaller tablet size offers advantages in terms of patient compliance and convenience.
References: 1. Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int J Pharm 2020, 586, 119560.
2. Oberoi, H.S.; Arce, F.; Purohit, H.S.; Yu, M.; Fowler, C.A.; Zhou, D.; Law, D. Design of a Re-Dispersible High Drug Load Amorphous Formulation. J Pharm Sci 2023, 112 (1), 250-263.
3. Armstrong, M.; Wang, L.; Ristroph, K.; Tian, C.; Yang, J.; Ma, L.; Panmai, S.; Zhang, D.; Nagapudi, K.; Prud'homme, R.K. Formulation and Scale-Up of Fast-Dissolving Lumefantrine Nanoparticles for Oral Malaria Therapy. Journal of Pharmaceutical Sciences, 2023.
4. Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev 2006, 58 (15), 1688-713.
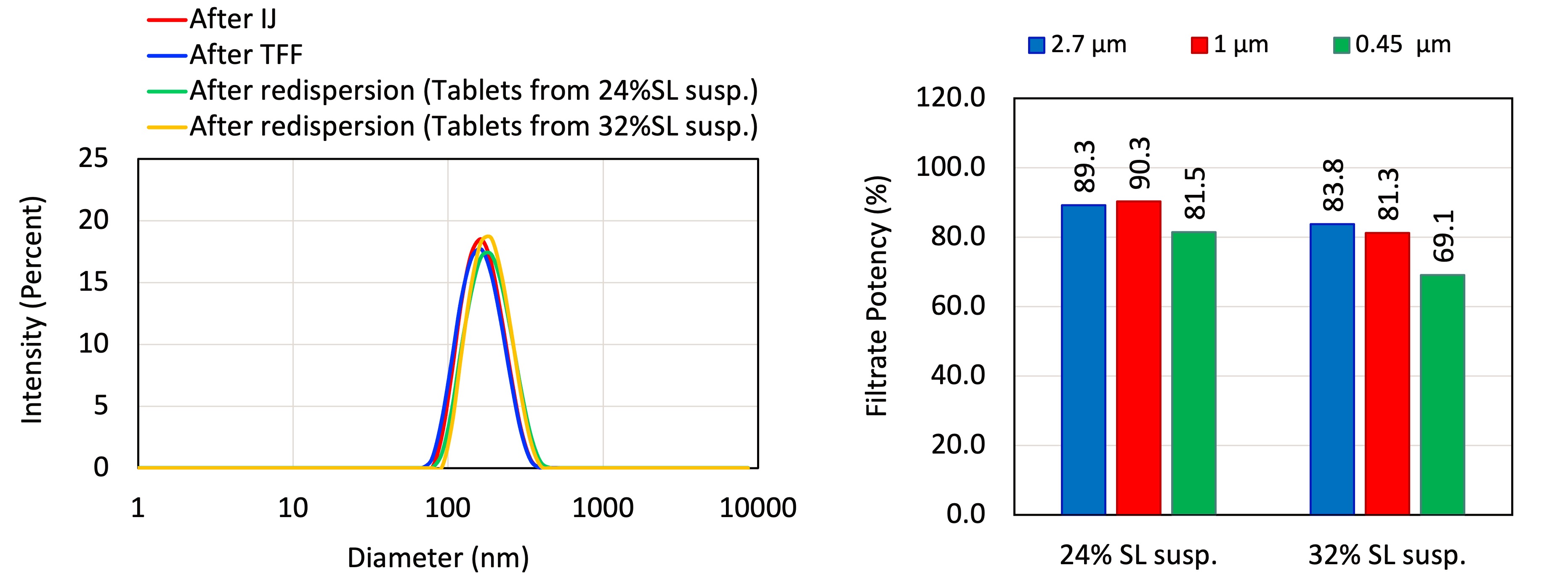 Figure 1. Size distribution of ANPs after formation by IJ mixing (red), after TFF (blue), and after re-dispersion from Tablets prepared from suspensions at 24% (w/v) SL (green) and 32% (w/v) SL (yellow). (B) Filtrate potency values for re-dispersed ABT-530 Tablets prepared from the pre-lyophilization suspensions at 24% (w/v) SL and 32% (w/v) SL. Values are normalized to corresponding unfiltered suspension control.
Figure 1. Size distribution of ANPs after formation by IJ mixing (red), after TFF (blue), and after re-dispersion from Tablets prepared from suspensions at 24% (w/v) SL (green) and 32% (w/v) SL (yellow). (B) Filtrate potency values for re-dispersed ABT-530 Tablets prepared from the pre-lyophilization suspensions at 24% (w/v) SL and 32% (w/v) SL. Values are normalized to corresponding unfiltered suspension control.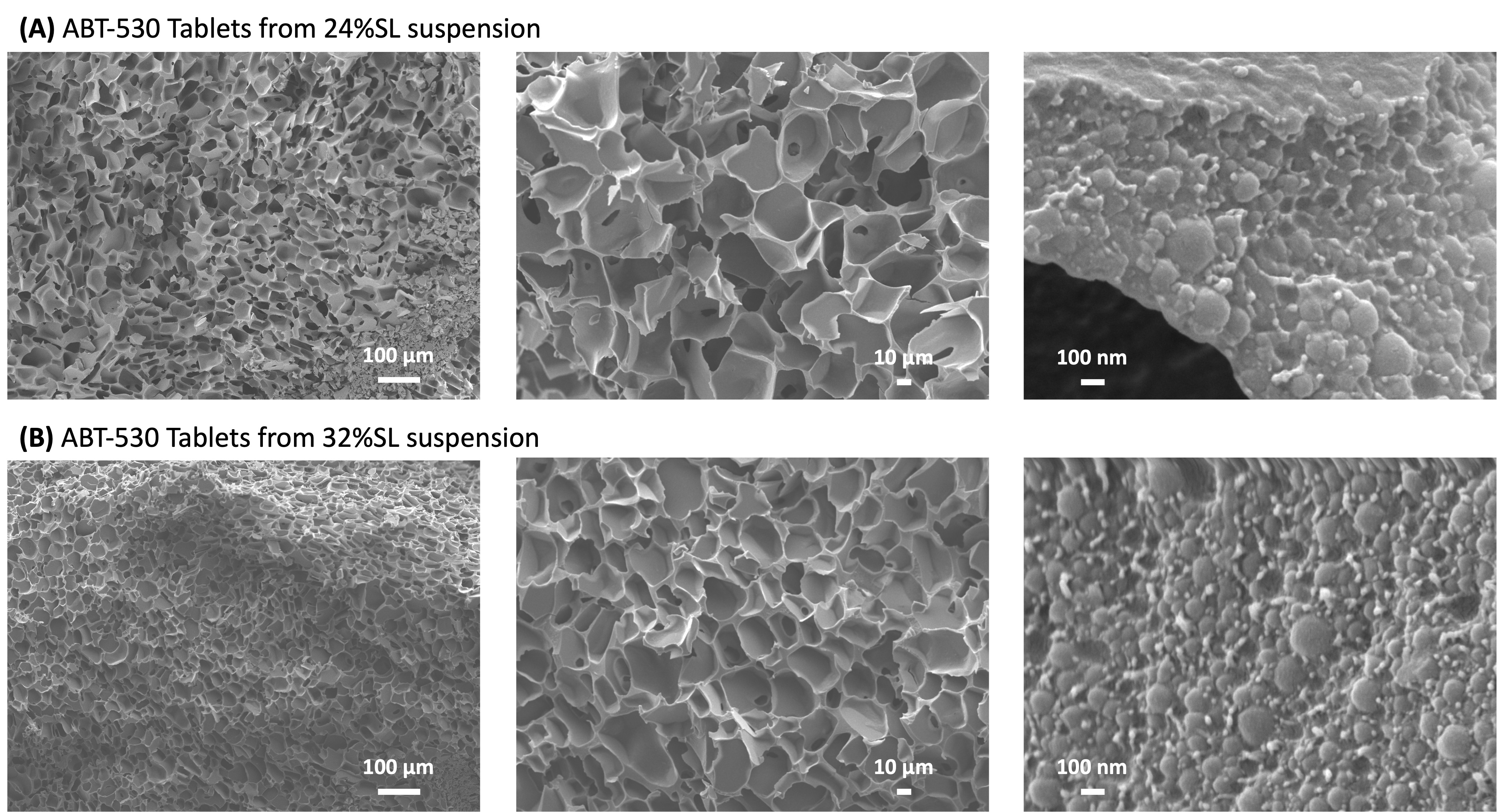 Figure 2. SEM images of ABT-530 Tablets prepared from the pre-lyophilization suspensions at (A) 24% (w/v) SL and (B) 32% (w/v) SL.
Figure 2. SEM images of ABT-530 Tablets prepared from the pre-lyophilization suspensions at (A) 24% (w/v) SL and (B) 32% (w/v) SL. 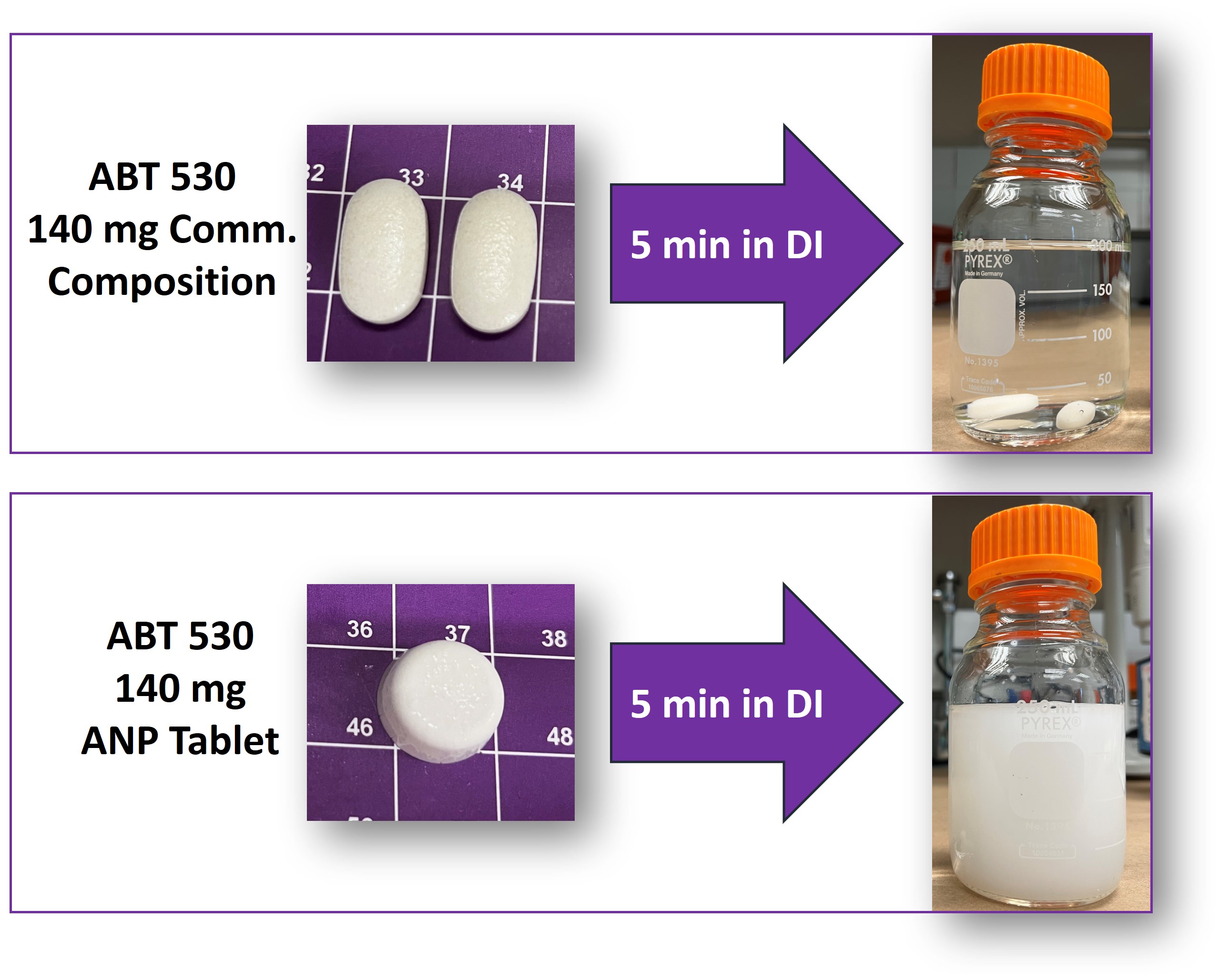 Figure 3. Comparison of re-dispersibility of a 140 mg ABT-530 commercial composition and ABT-530 ANP Tablets. Higher DL (60% (w/v)) resulted in a reduction in tablet size. In addition, ABT-530 ANP Tablets demonstrated the ability to re-disperse in water within less than 5 minutes.
Figure 3. Comparison of re-dispersibility of a 140 mg ABT-530 commercial composition and ABT-530 ANP Tablets. Higher DL (60% (w/v)) resulted in a reduction in tablet size. In addition, ABT-530 ANP Tablets demonstrated the ability to re-disperse in water within less than 5 minutes.