Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(M1130-09-62) A Continuous Future: Demonstrating Proof of Concept for Continuous Post-Drying of Pharmaceutical Spray Dried Intermediates
Monday, October 23, 2023
11:30 AM - 12:30 PM ET
- CB
Colton Bower, M.S. (he/him/his)
Merck & Co., Inc.
West Point, Pennsylvania, United States - CB
Colton Bower, M.S. (he/him/his)
Merck & Co., Inc.
West Point, Pennsylvania, United States - SF
Seth Forster, Ph.D. (he/him/his)
Merck & Co., Inc.
West Point, Pennsylvania, United States - ED
Erin Dippold, MEM (she/her/hers)
Merck & Co., Inc.
Rahway, New Jersey, United States - HW
Hailey Williams, B.S. (she/her/hers)
Merck & Co., Inc.
Rahway, New Jersey, United States - PB
Peter Brush, B.S. (he/him/his)
Merck & Co., Inc.
West Point, Pennsylvania, United States - AH
Abbe Haser, Ph.D. (she/her/hers)
Organon & Co., Inc.
Jersey City, New Jersey, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Spray drying is a common technique in the pharmaceutical industry for formulating amorphous solid dispersions. As evidenced by the use of this process in other industries, spray drying is a fundamentally continuous process, however, it is commonly executed in a batch production format in the pharmaceutical industry due to limitations in solution preparation, post-drying, cleaning, and other considerations. Converting to a more fully continuous operation would offer several attractive benefits for pharmaceutical manufacture, including reduced cycle times, improved yields, and more robust product quality. The intent of this work was to establish proof of concept for a continuous post-drying operation in order to enable incremental advancement towards fully continuous operation.
Methods: Placebo spray dried intermediate (SDI) was generated at 4 different operating conditions using a GEA Mobile Minor® spray dryer and commonly used pharmaceutical polymer and solvent, hypromellose acetate succinate and acetone, respectively. “Wet” SDI (SDI not subjected to post-drying) was collected and sealed in hermetic containers to maintain elevated levels of residual solvent. Continuous drying evaluations were performed using the L.B. Bohle QbCon® 1 continuous fluid-bed drying platform. Samples were collected from the outlet of the dryer and sealed in crimp-capped glass vials to maintain moisture and residual solvent levels. Physical characterization of the SDI included bulk and tapped density measurements per USP < 616 > using a VanKel Tap Density Tester and particle size distribution using a Sympatec RODOS QicPic dynamic image analysis with measurements in triplicate. Residual solvent levels in the SDI were measured offline by headspace gas chromatography using an Agilent 7890B. At-line measurements were performed by near infrared using a Sentronic SentroPAT FO with a SentroProbe diffuse reflectance probe in order to establish a model for future use in on-line or at-line testing.
Results: Challenges in feeding and transportation of SDI through the QbCon® equipment were encountered due to the physical attributes of the material (e.g. low bulk density, small particle size). Tuning of the dryer operating parameters and control of environmental conditions enabled successful transport of product through the dryer. Systematic variation of the key dryer operating parameters, solids feed rate, dryer speed, dryer temperature, and dryer airflow, were conducted across 25 trials in order to identify both conditions that result in successful drying as well as edges of failure. High feed rates (16 g/min) in combination with short residence time (~30 seconds) and low temperature (40-50°C) resulted in relatively high product residual solvent levels (6500 – 10000 ppm). Increasing product residence time (by decreasing solids feed and/or dryer speed) or increasing dryer temperature resulted in decreasing final product residual solvent level in a predictable manner. Trends between dryer operating parameters and resulting residual solvent levels demonstrate viability for establishing robust process control strategies. Comparison of product physical attributes (density and particle size) demonstrated no or negligible change in comparison to the pre-dried product indicating that use of continuous drying will not significantly alter product attributes and potentially enable transition of batch drying to continuous drying without requiring extensive re-formulation or downstream drug product optimization. Throughout the trials, > 99%w of the product used was recovered, demonstrating opportunity for significant yield improvements compared to traditional batch post-drying equipment.
Conclusion: The proof of concept for continuous post-drying of spray dried intermediate demonstrated in this study lays the groundwork for additional investigation into the technology and establishes a line of sight to enabling a fully continuous manufacturing process for a solid oral dosage drug product that incorporates a spray dried amorphous dispersion. Capability to remove residual solvents below ICH (International Council for Harmonisation) Q3C recommended limits using residence times of one minute or less also demonstrate significant opportunities for cycle time reduction in pharmaceutical processing. This work prompts the need for further investigations into continuous drying at larger scales as well as closer investigation of the risks to attributes of active compound containing amorphous dispersions subjected to continuous drying conditions.
Acknowledgements: Mike Fazio, Imre Homolya, Martin Hack, and Robin Meier from L.B. Bohle, LLC for their support in evaluations using the QbCon®1 continuous twin screw granulator. Shalisa Oburn, Sergio Sanchez, and Traci Scirbona for providing analytical and manufacturing support.
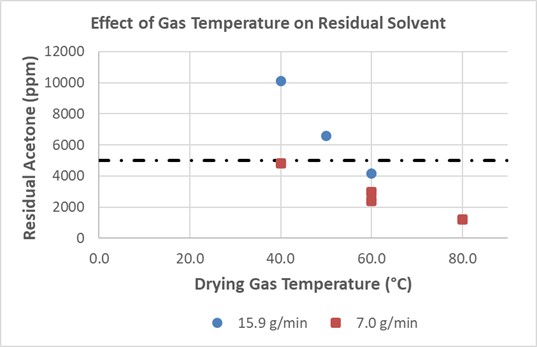 Figure 1. Effect of QbCon®1 operating parameters on residual solvent levels in the SDI product. Target acceptance criteria can be achieved by tuning equipment operation, demonstrating viability of establishing a robust control strategy.
Figure 1. Effect of QbCon®1 operating parameters on residual solvent levels in the SDI product. Target acceptance criteria can be achieved by tuning equipment operation, demonstrating viability of establishing a robust control strategy. 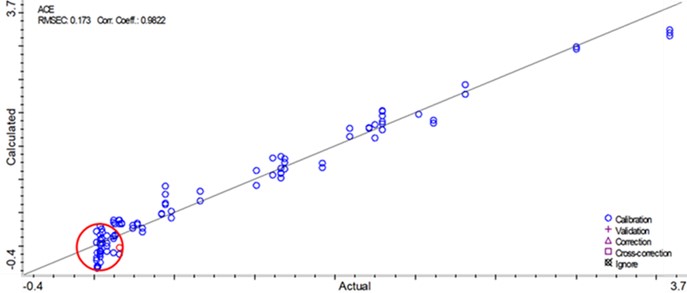 Figure 2. NIR model predictions for residual solvent compared to residual solvent levels measured by headspace gas chromatography. Good model fit and linearity were demonstrated for residual solvent levels greater than approximately 0.1%w/w.
Figure 2. NIR model predictions for residual solvent compared to residual solvent levels measured by headspace gas chromatography. Good model fit and linearity were demonstrated for residual solvent levels greater than approximately 0.1%w/w.