Formulation and Delivery - Biomolecular
Category: Late Breaking Poster Abstract
(M1130-12-77) Stabilization and Formulation Development of Lipid Nanoparticles (LNPs) for Nucleic Acid-Based Therapeutics
- LL
Lan Lan, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States - LL
Lan Lan, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States - DT
Daniel Turner, B.S.
Catalent Pharma Solutions
Bloomington, Indiana, United States - XS
Xuehan Shi, M.S.
Catalent Pharma Solutions
Bloomington, Indiana, United States - ZS
Zaneer Segu, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States - CS
Caleb Starr, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States - DH
Dawn Huffman, B.S.
Catalent Pharma Solutions
Bloomington, Indiana, United States - TS
Todd Stone, Ph.D. (he/him/his)
Catalent Pharma Solutions
Bloomington, Indiana, United States - LZ
Liling Zhang, M.S.
Catalent Pharma Solutions
Bloomington, Indiana, United States - LX
Lun Xin, M.S.
Catalent Pharma Solutions
Bloomington, Indiana, United States - YL
Yunsong Li, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States - JZ
Jingtao Zhang, Ph.D.
Catalent Pharma Solutions
Bloomington, Indiana, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: mRNA-based therapeutics have emerged as a powerful therapeutic modality after the approval of mRNA-based COVID vaccines. Since then, the technology has rapidly expanded into precision medicine and gene editing and is poised to significantly impact human health. As the major carrier for mRNA-based therapeutics, lipid nanoparticles (LNPs) have been studied extensively for their delivery performance. Yet, a thorough understanding of the stability of LNP-based therapeutics is lacking. As a result, the stability of these therapeutics remains a challenge for pharmaceutical use, as shown by the stringent handling procedure and deep-freezing conditions (-80 °C) for storage. Similarly, little literature exists regarding formulation excipients suitable for LNP stabilization. To address this industry-wide issue, we studied different aspects of LNP, including the instability mechanism, degradation pathways, and formulation excipients. In addition, we explored ways to improve the stability of the LNP product.
Methods: LNPs containing mRNA or polyA were assembled using microfluidic mixing, purified using dialysis, and prepared indifferent excipient matrixes. LNPs were subject to different stress conditions and further analyzed using modern analytical methods, including CE (capillary electrophoresis), Ribogreen assay, and DLS (dynamic light scattering). In addition, advance analysis using tools such as HPLC-CADEAF4 (Electrical asymmetrical flow field-flow fractionation) and NTA (Nanoparticle Tracking Analysis) for LNP characterizations were also used to probe the instability mechanism.
Results: We show that freeze-thaw, agitation, and lyophilization can induce significant degradation in LNP. Nevertheless, both the type and level of excipients can play an important role in determining the stability of LNP products. We demonstrate, for example, that a few selected cryoprotectants performed best under freeze-thaw conditions in maintaining LNP stability compared to other excipients, as shown by the least changes in LNP diameter and percent of encapsulation (Figure 1). Furthermore, chemical degradation of mRNA within LNP appears to be rate limiting in determining drug product shelf life.
Conclusion: LNP product is unique in its instability behavior compared with other biological molecules and requires special formulation approaches. Different excipients and combinations are needed to preserve the stability of LNP for mRNA delivery. We have established a key capability focusing on LNP formulation and analytical development to screen for stable formulations and characterize the stability of LNP/mRNA. Further development will aid the field in establishing platform approaches for LNP formulation and support the development of this rapidly growing modality.
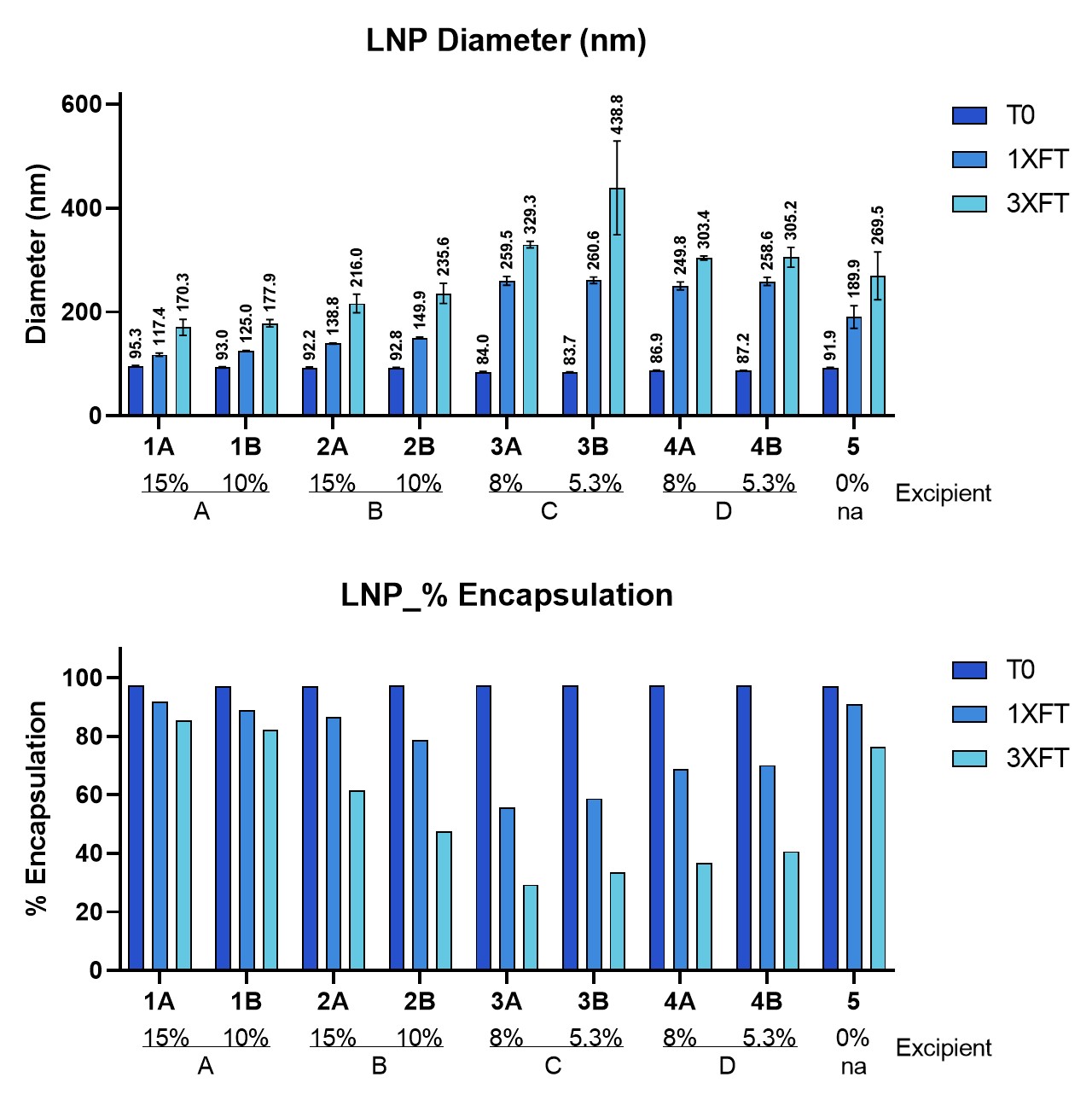 Selected cryoprotectants help maintaining LNP stability under freeze-thaw (FT) conditions. LNP samples with different cryoprotectants were frozen at -80°C for overnight followed by thawing at 2-8°C for 4-6 hours. Samples were analyzed following one (1) and three (3) FT cycles. Cryoprotectant A showed best performance under freeze-thaw conditions in maintaining LNP stability compared to other excipients, as shown by the least changes in LNP diameter (Top) and percent of encapsulation (Bottom).
Selected cryoprotectants help maintaining LNP stability under freeze-thaw (FT) conditions. LNP samples with different cryoprotectants were frozen at -80°C for overnight followed by thawing at 2-8°C for 4-6 hours. Samples were analyzed following one (1) and three (3) FT cycles. Cryoprotectant A showed best performance under freeze-thaw conditions in maintaining LNP stability compared to other excipients, as shown by the least changes in LNP diameter (Top) and percent of encapsulation (Bottom).