Formulation and Delivery - Chemical
Category: Late Breaking Poster Abstract
(M1030-03-18) Formulation of Omeprazole Minitablets with Delayed Release: A Seal and Enteric Polymer Coating Approach
Monday, October 23, 2023
10:30 AM - 11:30 AM ET
- MJ
Ming Ji, Ph.D.
BASF Corporation
Tarrytown, New York, United States - MJ
Ming Ji, Ph.D.
BASF Corporation
Tarrytown, New York, United States - KC
Kun Chen, Ph.D.
BASF Corporation
Tarrytown, New York, United States - RM
Ramesh Muttavarapu, M.S.
Thermo Fisher Scientific
Cincinnati, Ohio, United States - GM
Gyan Mishra, Ph.D.
Thermo Fisher Scientific
Cincinnati, Ohio, United States - BG
Bhim Gadal
Thermo Fisher Scientific
Cincinnati, Ohio, United States - KM
Kavya Muddsani
Thermo Fisher Scientific
Cincinnati, Ohio, United States - NS
Nitin Kumar Swarnakar, Ph.D.
BASF Corporation
Tarrytown, New York, United States - ST
Sandip Tiwari, Ph.D.
BASF Corporation
Tarrytown, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The primary objective of this study was to develop delayed-release omeprazole minitablets using a direct compression method and ensure the stability of drug by applying a sub-coating of instant release polyethylene glycol- polyvinyl alcohol graft copolymer followed by the enteric coating process using methacrylic acid-ethyl acrylate co-polymer (1:1) dispersion using the enteric coating polymer.
Methods: Compatibility study of Omeprazole and coating polymer solutions: Omeprazole (Teva Pharmaceuticals Industries Ltd) was dissolved in ethanol with a 0.2 mg/mL concentration. The 6% (w/w) polyethylene glycol- polyvinyl alcohol graft copolymer and methacrylic acid-ethyl acrylate co-polymer (1:1), the enteric coating polymer (Kollicoat® MAE 100P: pre-neutralized spray dried form of Kollicoat® MAE 30D) solutions were prepared by adding the polymers to ethanol:water (50:50, v/v) and mixing with a magnetic stirrer for ~1 hr. The 0.02 mg/mL omeprazole solutions were prepared by mixing 5 mL of the 0.2 mg/mL omeprazole solution with 45 mL 0.1 N NaOH, acetic acid (6% w/w) in ethanol:water (50:50, v/v), ethanol:water (50:50, v/v) and ethanol:water (50:50, v/v) with 6% (w/w) polymers. All the solutions were stored at ambient temperature and wrapped with aluminum foil to prevent degradation from light. The stability of omeprazole was monitored by UV-vis absorption spectra on Pion’s fiber optic UV detection system, the Rainbow R6. Tablet formulation and coating: The directly compressible tablet blend was prepared by screening and mixing the microcrystalline cellulose, spray dried mannitol, povidone K30, silicon dioxide colloidal and magnesium stearate ingredients. The blend was compressed into uncoated minitablets on Picola Press (RIVA S.A., Agentina) using 2.00 mm standard concave round shaped punch in 6-9 mm cam at precompression force 0.2 kN and main compression force 4-6 kN. The minitablets were sub-coated at 8% weight gain using 20% coating solution of the seal coating polymer (Kollicoat® IR) without plasticizer in LDCS coater (with 0.5 L pan). Finally, minitablets were enteric coated in the same instrument at various coating levels (8%, 12% and 20% coating weight gain, or 3, 5 and 8.5 mg/cm2 coating levels) using 20% coating dispersion containing the enteric coating polymer, talc, triethyl citrate. Dissolution studies: The in -vitro dissolution of minitablets were performed using USP type II apparatus using 750 ml of 0.1 M HCl for first 2h followed by 1000 mL phosphate buffer pH 6.8 for 3h at the paddle speed of 100 rpm at 37± 0.5°C.
Results: Omeprazole was stable in alkaline media and ethanolic/water (50:50, v/v) (Figure 1A and Figure 1C and λ 305 nm / λ276 nm = 1.768 within as per pharmacopeia requirement) but degraded rapidly in low pH acetic acid media (Figure 1B, bathochromic shift and increase absorption by 56% at 305 nm).(1,2) Acidic polymethacrylate solution of the enteric coating polymer also degraded the drug (Figure 1D; bathochromic shift and increase absorption by 2% at 305 nm) but to a lesser extent as compared to the acetic acid solution because the acidic groups of polymethacrylate were shielded by the long polymer chains.(2) In contrast, omeprazole was stable in the presence of the seal coating polymer (Kollicoat® IR) solution (Figure 1E). Therefore, a seal coating with the seal coating polymer was applied to minitablet to protect omeprazole degradation during enteric coating process. Uncoated minitablets (each weighing 7.4 mg and containing 1 mg of omeprazole) exhibited adequate mechanical strength ( >1.7 mPas) and passed the friability test, indicating their suitability for the coating process. The seal coating polymer was selected over conventional HPMC 3 mPas polymer because it was intrinsically free of peroxides, more elastic even without plasticizer (data not shown), and easy to coat (requires product temperature >15ºC). The seal coating polymer solution (20%w/w) showed low viscosity ( < 100mPas), leading to faster and stable film coating process to achieve 14-15% coating weight gain (5.8 – 6.2 mg/cm2) in the minitablet (30 min coating time for 50,000 tablets). Later, the enteric coating properties of the enteric coating polymer were investigated on seal coated minitablets containing omeprazole. Among various coating levels (3-8.5 mg/cm2), minimum 5 mg/cm2 coating exhibited no drug release at gastric pH ( < 1.2) within 120 minutes, while similar and complete ( >85%) release was obtained at a pH of 6.8 within 90 min (Figure 2). Therefore, it is expected that inter batch variability during the scale up batches will not have any impact on dissolution profile.
Conclusion: A core omeprazole minitablets were developed using a direct compression method. The compatibility of omeprazole with the coating polymers was evaluated, revealing its stability in the seal coating polymer solution but instability in presence of the acidic enteric coating polymer. Therefore, delayed release omeprazole minitablets were successfully prepared by application of a sub-coating of instant release seal coating polymer (~6 mg/cm2) followed by the enteric coating polymer coating (3 mg/cm2 to 8.5 mg/cm2).
References: [1] A. Pilbrant, Development of an oral formulation of omeprazole. Scand J Gastroenterol, 1985, 20:113–119.
[2] A. Riedel, C. S. Leopold, Quantification of omeprazole degradation by enteric coating polymers: an UV-VIS spectroscopy study. Pharmazie, 2005, 60:126-130
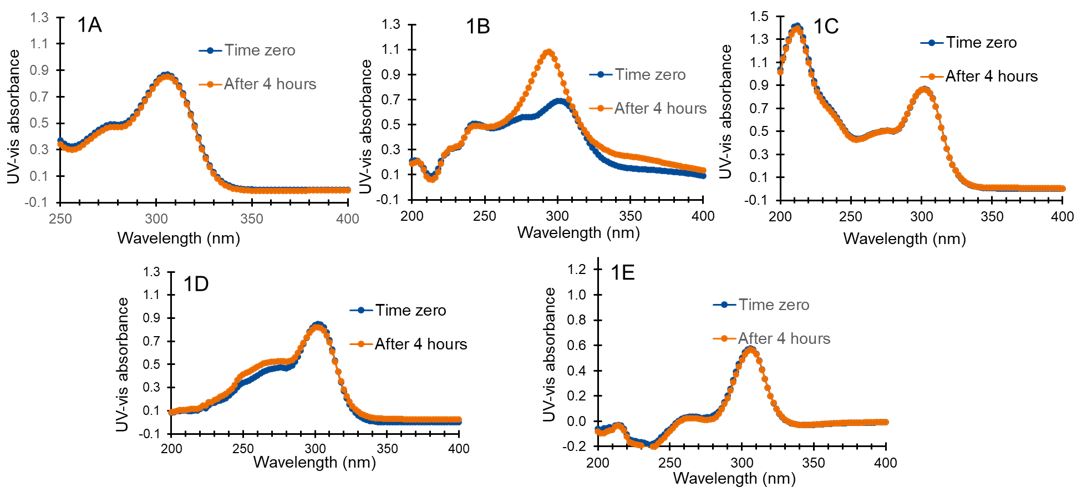 Figure 1. UV vis spectra of omeprazole (0.02 mg/mL) in different solvents at two different time points. A) 0.1 N NaOH; B) Acetic acid (6%, w/w) in ethanol/water (50:50, v/v); C) Ethanol/water (50:50, v/v); D) The enteric coating polymer (6%, w/w) in ethanol/water (50:50, v/v); E) The seal coating polymer (6%, w/w) in ethanol/water (50:50, v/v).
Figure 1. UV vis spectra of omeprazole (0.02 mg/mL) in different solvents at two different time points. A) 0.1 N NaOH; B) Acetic acid (6%, w/w) in ethanol/water (50:50, v/v); C) Ethanol/water (50:50, v/v); D) The enteric coating polymer (6%, w/w) in ethanol/water (50:50, v/v); E) The seal coating polymer (6%, w/w) in ethanol/water (50:50, v/v).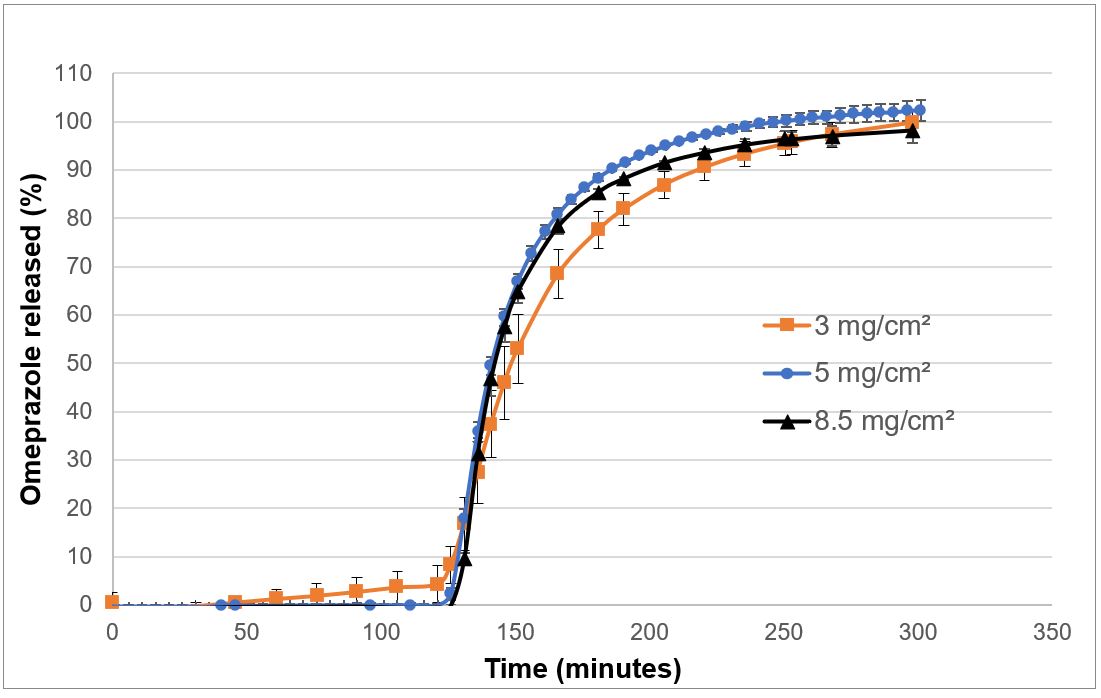 Figure 2: Dissolution of enteric-coated omeprazole minitablets in 0.1 N HCl media up to 120 min (Acid stage) followed by pH 6.8 phosphate buffer (buffer stage) using a USP Type 2 apparatus maintained at a 100-rpm paddle speed at 37 °C.
Figure 2: Dissolution of enteric-coated omeprazole minitablets in 0.1 N HCl media up to 120 min (Acid stage) followed by pH 6.8 phosphate buffer (buffer stage) using a USP Type 2 apparatus maintained at a 100-rpm paddle speed at 37 °C.