Manufacturing and Analytical Characterization - Chemical
Category: Late Breaking Poster Abstract
(M0930-09-59) CryoTEM Imaging of Onivyde for Precise Reverse Manufacturing
Monday, October 23, 2023
9:30 AM - 10:30 AM ET

Jingyao Gan, M.S. (she/her/hers)
postdoc
University of Michigan
Ann Arbor, Michigan, United States
Jingyao Gan, M.S. (she/her/hers)
postdoc
University of Michigan
Ann Arbor, Michigan, United States- JL
Jing Liang, Ph.D.
University of Michigan
ANN ARBOR, Michigan, United States - VJ
Vivian Juang, Ph.D.
University of Michigan
ANN ARBOR, Michigan, United States 
Anna Schwendeman, PhD (she/her/hers)
Hans W. Vahlteich Professor of Pharmacy
University of Michigan
Ann Arbor, Michigan, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Onivyde, an intravenous irinotecan liposomal injection, has emerged as a successful treatment option for post-gemcitabine metastatic adenocarcinoma of the pancreas since its approval in 2015 by the FDA and achieved remarkable annual sales of $1.8 billion last year [1, 2]. However, the expensive price and complex manufacturing process limit access to a larger population of patients. More importantly, while the patent will lose validation soon, there is not enough reference information on its critical quality attributes (CQAs). In this work, we observed four batches of Onivyde using cryo-transmission electron microscopy (cryoTEM) imaging to establish a set of physical parameters for guiding and evaluating the reproduction of the manufacturing process and to pave the way for possible future development of generic versions of liposomal irinotecan.
Methods: Onivyde was purchased from the University of Michigan Health System Research Pharmacy. The particle size was measured by dynamic light scattering (DLS) using a Malvern ZetaSizer Nano ZS and the morphology of Onivyde was visualized by a cryogenic transmission electron microscope (cryoTEM). Home-made liposomal irinotecan was produced following the manufacturing process [3] in Figure 3, and its hydrated particle size and size distribution were measured by DLS. CryoTEM images were processed in ImageJ software. The data were analyzed using GraphPad Prism 8.
Results: CryoTEM images showed a prolate spheroid or spheroid shape of Onivyde and its nanostructure in detail (Figure 1). Different from the uniformity of Doxil liposomal structure [4], Onivyde presented a more complex existence in which most liposomes are unilamellar vehicles with or occasionally without drug inside (Figure 1, blue arrows), and a small part has multilamellar vehicles (Figure 1, red arrows) or multivesicular vehicles (Figure 1, white arrows). Drug crystal bundles are encapsulated in the inner compartment of liposome straight or twisted, longitudinal or cross-sectional. In multivesicular vehicles, the number of liposomes in most cases is two and rarely over two (Figure 1 A2, B1, C1, D2 white arrows). Even though the non-unilamellar vehicle rate of 3rd batch of Onivyde is lower (25.7% in Figure 2D) than that in other batches where deep research is needed to clarify the possible reasons, there is no significant difference among their long diameters and short diameters (Figure 2D). To figure out the practical shape of Onivyde, a 3D observation mode will be conducted to distinguish if there is a coexistence of prolate spheroid and spheroid. In cryoTEM analysis (Figure 2D), the proportion of the multilamellar vehicles or multivesicular vehicles is between 25-36%. The shapes of Onivyde in CryoTEM images reflected there being a special ratio between lipids and the volume of hydrating solution which created the non-unilamellar vehicle phenomenon affecting the drug-loading, drug-release behavior, even efficacy in vivo, etc. Based on cryoTEM imaging, the home-made Onivyde could be evaluated, and the manufacturing process will be further verified for precise reproduction of process. In Figure 3, the first trial of Onivyde was carried out following the manufacturing processes and the intensity size of original formulation was 106.7± 0.8 nm, which showed no significant difference with 1st batch of Onivyde and was slightly smaller than other batches of Onivyde. In the following plans, more comprehensive evaluations will be conducted on the home-made liposomal irinotecan including incompletely the quantification of lipids and drug, drug encapsulation efficiency based on the methods we established [5], and the fine nanostructures of formulation.
Conclusion: We established a set of physical parameters of Onivyde using cryoTEM imaging to guide the reproduction of manufacturing process which will probably pave the way for further evaluation of batch-to-batch variability of approved product and development of generic versions of liposomal irinotecan.
References: 1. Frampton JE. Liposomal Irinotecan: A Review in Metastatic Pancreatic Adenocarcinoma Drugs. 2020;80(10):1007-1018.
2. https://www.fiercepharma.com/pharma/ipsens-onivyde-proves-its-worth-metastatic-pancreatic-ductal-adenocarcinoma
3. Drummond DC, 2018, Liposomal irinotecan preparations, US 2018/0110771 A1, Ipsen Biopharm Ltd
4. Nordström R, Zhu L, Härmark J, et al. Quantitative Cryo-TEM Reveals New Structural Details of Doxil-Like PEGylated Liposomal Doxorubicin Formulation. Pharmaceutics. 2021;13(1):123.
Acknowledgements: Research reported in this publication was supported by the University of Michigan Cryo-EM Facility (U-M Cryo-EM). U-M Cryo-EM is grateful for support from the U-M Life Sciences Institute and the U-M Biosciences Initiative. This study is supported by United States (U.S.) Food and Drug Administration (FDA) Grant U18FD007054.
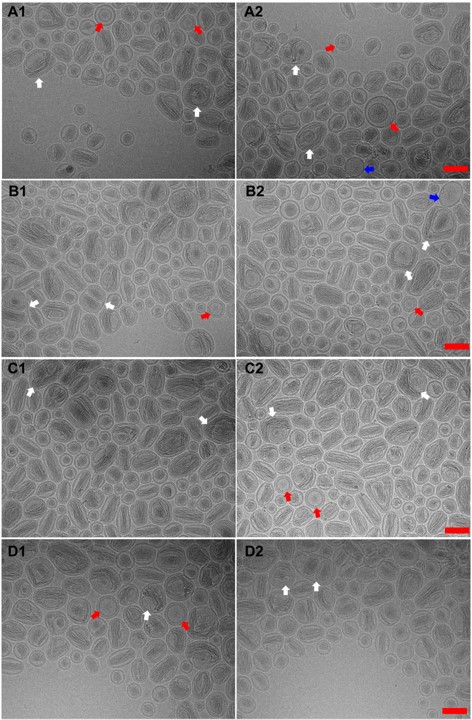 Figure 1. CryoTEM images of different batches of Onivyde. White arrows refer to multivesicular vehicles. Red arrows indicate multilamellar vehicles. Blue arrows point out unilamellar vehicles with little drug crystal inside. A1-A2, B1-B2, C1-C2 and D1-D2 are representative cryoTEM images of 1st batch, 2nd batch, 3rd batch, 4th batch of Onivyde respectively. Scale bat, 100 nm.
Figure 1. CryoTEM images of different batches of Onivyde. White arrows refer to multivesicular vehicles. Red arrows indicate multilamellar vehicles. Blue arrows point out unilamellar vehicles with little drug crystal inside. A1-A2, B1-B2, C1-C2 and D1-D2 are representative cryoTEM images of 1st batch, 2nd batch, 3rd batch, 4th batch of Onivyde respectively. Scale bat, 100 nm.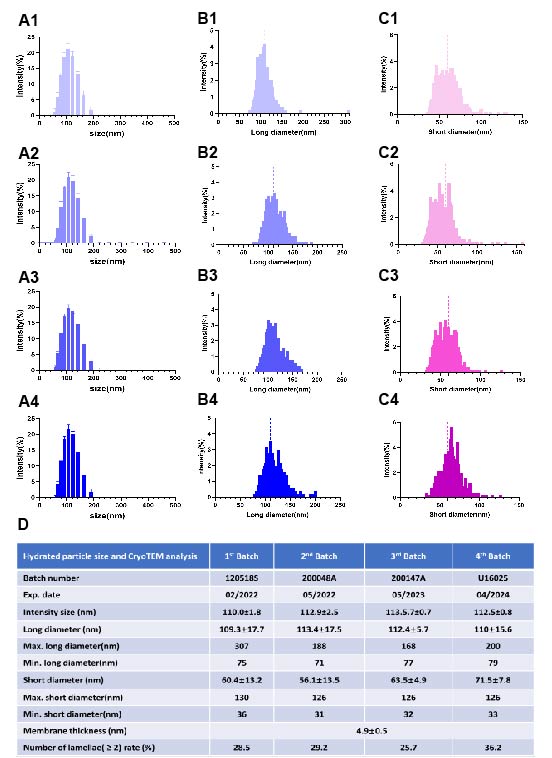 Figure 2. Hydrated particle size distribution and cryoTEM analysis of 4 batches of liposomal Onivyde formulations. A1-A4, intensity size distribution of 1st to 4th batch of Onivyde respectively. B1-B4, long diameter distribution of 1st to 4th batch of Onivyde respectively (n>500). C1-C4, short diameter distribution of 1st to 4th batch of Onivyde respectively (n>500). D, hydrated particle size and cryoTEM analysis. The number of observed and analyzed particles is over 500. All values are presented as mean ± SD(n≥3).
Figure 2. Hydrated particle size distribution and cryoTEM analysis of 4 batches of liposomal Onivyde formulations. A1-A4, intensity size distribution of 1st to 4th batch of Onivyde respectively. B1-B4, long diameter distribution of 1st to 4th batch of Onivyde respectively (n>500). C1-C4, short diameter distribution of 1st to 4th batch of Onivyde respectively (n>500). D, hydrated particle size and cryoTEM analysis. The number of observed and analyzed particles is over 500. All values are presented as mean ± SD(n≥3).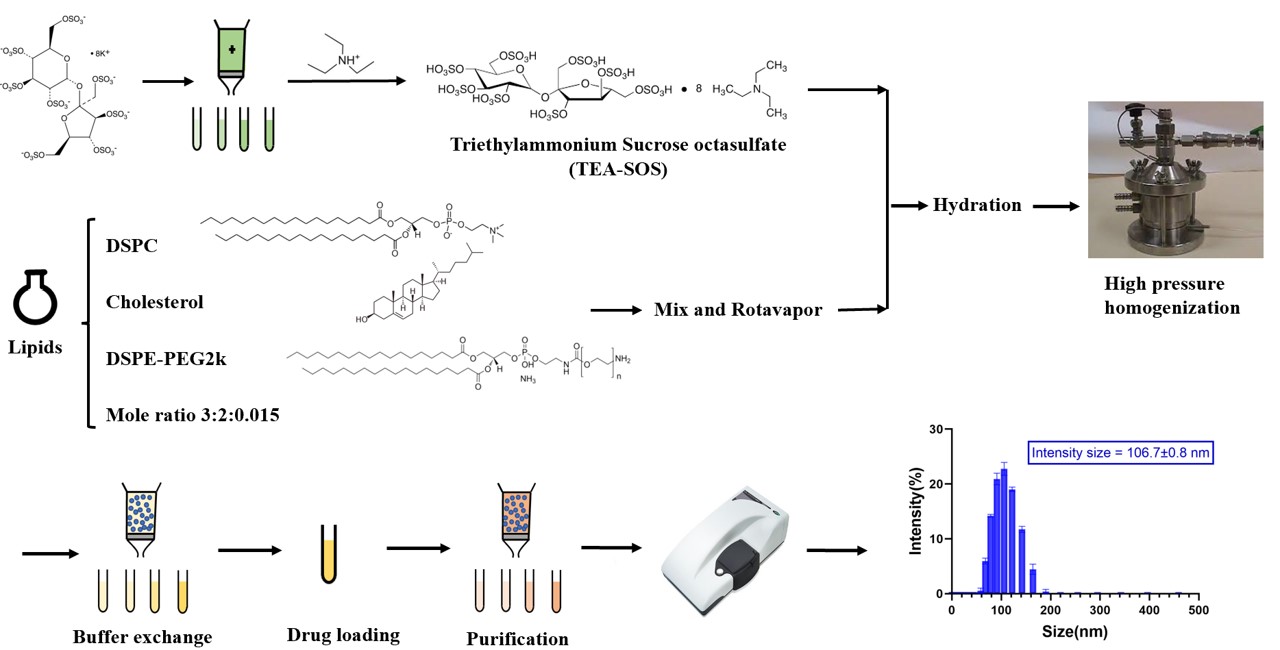 Figure 3. Manufacturing of Onivyde and the particle size and size distribution of first trial.
Figure 3. Manufacturing of Onivyde and the particle size and size distribution of first trial.