Preclinical and Translational Sciences - Biomolecular
Category: Late Breaking Poster Abstract
(M0930-10-65) Prospectively Assessing the Immunogenic Risk of Potential Synthetic Peptide Impurities In Silico Using the What-if Machine (WhIM)
Monday, October 23, 2023
9:30 AM - 10:30 AM ET
- RN
Riley Nolan
EpiVax, Inc.
Providence, Rhode Island, United States - AM
Aimee Mattei, M.S.
EpiVax, Inc.
Providence, Rhode Island, United States - MA
Matthew Ardito
EpiVax, Inc.
Providence, Rhode Island, United States - WM
William Martin
EpiVax, Inc.
Providence, Rhode Island, United States 
Brian Roberts, Ph.D.
Scientific Director, Preclinical Immunology
EpiVax, Inc.
Providence, Rhode Island, United States
Anne DeGroot, M.D.
CEO/CSO
EpiVax, Inc.
Providence, Rhode Island, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Ensuring drug safety and efficacy is of utmost importance for bringing novel and generic peptide drug products to market. Assessing the immunogenic potential of a given peptide drug product is a key element to safety and efficacy evaluations. Assessing the immunogenicity risk of the active pharmaceutical ingredient (API) as well as the associated impurities is critical to evaluating the safety of the product. Impurities found in synthetic peptide drugs can result from failures in the peptide synthesis process or may be the result of instability after formulation. The modifications in these sequence-related impurities can alter the peptide’s affinity for binding to human leukocyte antigen (a prerequisite for T cell-dependent immune response) or can alter the humanness of T cell epitope content relative to the API. Impurities that introduce foreign new T cell epitope content can increase the overall immunogenicity risk profile of the drug product, even when present in small amounts. The What-if Machine (WhIM) is an in silico algorithm that prospectively predicts potential impurities that could impact the immunogenicity of a given API.
Methods: The What-if Machine (WhIM) is an algorithm that, for a given input peptide sequence, models (in silico) nearly all impurities that may occur during peptide manufacturing and storage, such as amino acid deletions, insertions, racemization, and side chain modifications. WhIM generates a comprehensive list of thousands of theoretical impurities, depending on sequence length. The generated impurity sequences are scored and ranked with the well-established immunoinformatics tools, EpiMatrix for immunogenicity and JanusMatrix for tolerance, at both an overall and an impurity-specific level. EpiMatrix predicts T cell epitope content while JanusMatrix assesses the putative T cell epitopes for potential human homology-induced tolerance. A summarized immunogenicity risk profile for the novel or generic peptide drug and its WhIM-generated ranked list of theoretical impurities is produced.
Results: We performed WhIM analysis on a set of peptide drugs that are either currently on the market or are in late-stage clinical trials and provide impurity risk profiles for each. The WhIM impurity risk profiles suggest that some API peptides, like teriparatide, have a higher risk of generating immunogenic impurities than others. WhIM analysis examples for two generic peptide drugs, teriparatide and semaglutide, and the in vitro validation of selected WhIM-identified impurities is provided. From the impurity risk profiles, in general, teriparatide carries a higher risk of generating immunogenic impurities compared to semaglutide. In T cell assays measuring responses in naïve donor PBMCs, high risk teriparatide impurities elicited an immune response in more donors than the API peptide at equal concentrations.
Conclusion: The WhIM algorithm could be used proactively to ensure proper manufacturing procedures are in place to limit the generation of high-risk impurities, saving resources in the effort to ensure the development of safe and effective novel or generic peptide therapeutics. From the comprehensive interactive list of generated impurities, the specific highest-risk impurities can be identified. Their immunogenic risk versus likelihood of occurrence can be weighed by the drug manufacturer based on the associated synthesis and storage procedures. WhIM is also a useful tool for regulators or sponsors to retrospectively investigate the immunogenic potential of specific impurities identified in the drug product. It is recommended that WhIM be used in conjunction with in vitro HLA binding and T cell assays, which serve to validate the predicted immunogenic sequences if they are in fact identified in the drug product.
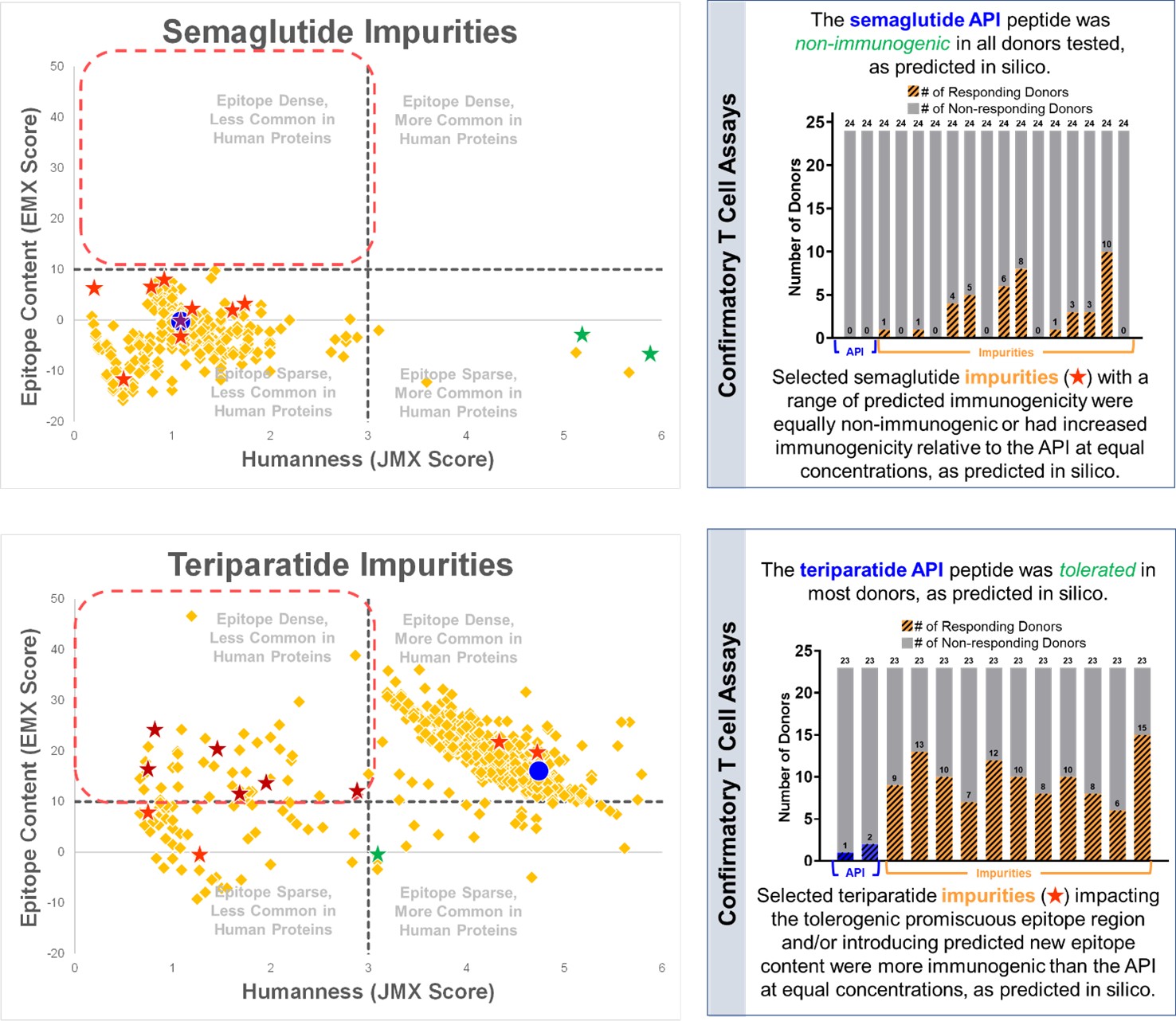 The WhIM-generated impurity risk profiles are shown on the left hand side for two peptide drugs - Semaglutide and Teriparatide. Impurities falling in the top left quadrant have the highest risk of impurity-induced immunogenicity. On the right hand side, the number of responding donors in T cell assays using naive donor PBMCs are shown for the APIs and selected WhIM-identified impurities.
The WhIM-generated impurity risk profiles are shown on the left hand side for two peptide drugs - Semaglutide and Teriparatide. Impurities falling in the top left quadrant have the highest risk of impurity-induced immunogenicity. On the right hand side, the number of responding donors in T cell assays using naive donor PBMCs are shown for the APIs and selected WhIM-identified impurities.