Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M1130-08-55) Effects of SDS Quality and Dissolution Medium Warm-Up Time on Dissolution Release of MRTX849 IR Tablets, 200 mg
.jpg)
Ken (Zhengkun) Yu, MS
Supervisor, Analytical Development
Thermo Fisher Scientific
Mississauga, Ontario, Canada.jpg)
Ken (Zhengkun) Yu, MS
Supervisor, Analytical Development
Thermo Fisher Scientific
Mississauga, Ontario, Canada- MC
Monica Choi, Ph.D.
Mirati Therapeutics
San Diego, California, United States - LF
Lee Fang, MS
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - LZ
Linus Zeng, MS
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - JJ
John Jiang, Ph.D.
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - NT
Nitin Tailor, MS
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - QA
Quazi Ahmed, MS
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - GC
Geoffrey P. Carr, Ph.D.
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada - WY
William Yu, MS
Patheon, Part of Thermo Fisher Scientific
Mississauga, Ontario, Canada
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: An HPLC dissolution method was successfully developed for MRTX849 (Adagrasib) IR tablets with pH 6.8 phosphate buffer plus 0.3% SDS (Sodium Dodecyl Sulfate) as medium. However, high variations were observed during method validation and routine stability sample testing. To get consistent dissolution results and profiles, a series of investigations were conducted. Experimental results demonstrated that SDS grade and dissolution medium warm-up time are critical for consistent dissolution results generation.
Methods: To prepare 18 L of dissolution medium, dissolve 118.26 g of Na2HPO4.7H2O and 63.36 g of NaH2PO4.H2O in 18 litres of DI water in a 20-litre clear carboy using a 3-inch stirring bar. Adjust pH to 6.80 ± 0.05 with NaOH or HCl. Sparge helium into the medium for at least 15 minutes. Transfer 54.0 grams of SDS and a 2-inch stirring bar into a 6-litre Erlenmeyer flask containing 2400 mL of degassed phosphate buffer, wash inner walls after adding SDS with 600 mL of degassed phosphate buffer. Cover the flask, set the stirring speed at approx. 280-300 rpm at beginning, after approx. 10 minutes, adjust the stirring speed to 200 rpm, keep the solution stirred steadily for overnight (NLT 16 hours). Transfer the 3000 mL of stock SDS solution into the carboy, rinse the Erlenmeyer flask at least four times with phosphate buffer solution, add rinsate into the same carboy, stir the bulk solution for 24 hours. Dispense 900 mL medium into each of the dissolution vessels, assemble the apparatus and equilibrate the dissolution medium to 37 ± 0.5 °C (record the temperature) for two hours with stirring at 75 rpm.
Results: High variations were found from historical data analysis of MRTX849 dissolution results generated with two grades of Fisher SDS (Cat # S529 and BP166, NF/FCC). To find the root cause of high variations, the following parameters and conditions were evaluated.
Filter bias, different filters were used for sampling.
Working standard solution preparation, same sample solutions were analyzed with two different working standard solutions prepared by two chemists.
Different dissolution systems.
Different dissolution bath temperatures at 36.5°C and 37.5°C.
Medium preparation procedures with deliberate changes, such as different stirring time for stock SDS solutions at 11 hours and 17 hours, and different sitting time of medium on bench, in which same medium was used for dissolution on day 0, day 1 and day 4.
Different grade of SDS, including Fisher SDS, ≥85.0% and Sigma SDS, ≥99.0% (GC).
The investigation concluded that SDS purity grade is a critical factor for MRTX849 dissolution. Difference was observed between Sigma SDS (Sigma-Aldrich SDS, 71729-500G, ≥98.0%, 71725-500G, ≥99.0%) and Fisher SDS (Cat # S529, ≥85.0% and BP166, ≥99%). The major difference between two reagents is that Sigma SDS dissolves fast in buffer. On the contrary, two grades of Fisher SDS take longer time to dissolve. Consistent dissolution results were obtained at a range of 86% to 93% released at 30 minutes (Q=80%) when Sigma SDS was used. However, higher variation of dissolution results as well as lower dissolution release values were observed at a range of 66% to 93% released at 30 minutes when two grades of Fisher SDS were used. Dissolution results and profiles from method validation and one of stability protocols are listed in Figure 1 and Figure 2. Exact same trend was observed from all other release and stability sample testing. Meanwhile, a very interesting phenomenon was observed during the execution of equivalency study of manual and automated dissolution systems for MRTX849 IR tablets dissolution. Dissolution profiles and %RSD at each time point are related to dissolution medium warm-up time in dissolution vessels. The results demonstrated that 2 hours warm-up time is necessary to get consistent dissolution profiles with low %RSD at each time point, and all results were confirmed on different dissolution systems, which are listed in Figure 3. The results showed that 0.5 hours medium warm-up time is not enough (%RSD > 3% at 20 and 30 minutes). Two hours medium warm-up time was addressed in the dissolution method for future automated and manual sampling dissolution testing. The potential mechanism is that it takes at least 2 hours to form consistent micelles of SDS in the medium for MRTX849 IR tablets dissolution testing.
Conclusion: To get consistent dissolution results and profiles of MRTX849 IR Tablets, high grade of Sigma SDS and 2 hours of medium warm-up time are critical for the MRTX849 IR tablets dissolution method. The method, CTMLP-4998, has been revised accordingly by adding the SDS catalogue number and medium warm-up time in vessels. Consistent dissolution profiles have been obtained on different dissolution systems by different scientists from development department to QC department in the past for more than one year. In addition, three certificates of analysis of Sigma SDS and Fisher SDS were reviewed, the differences between Sigma SDS and Fisher SDS will be further discussed in full poster.
.jpg) Figure 1, Dissolution Results Generated with Fisher SDS from December 2020 to Jun 2021
Figure 1, Dissolution Results Generated with Fisher SDS from December 2020 to Jun 2021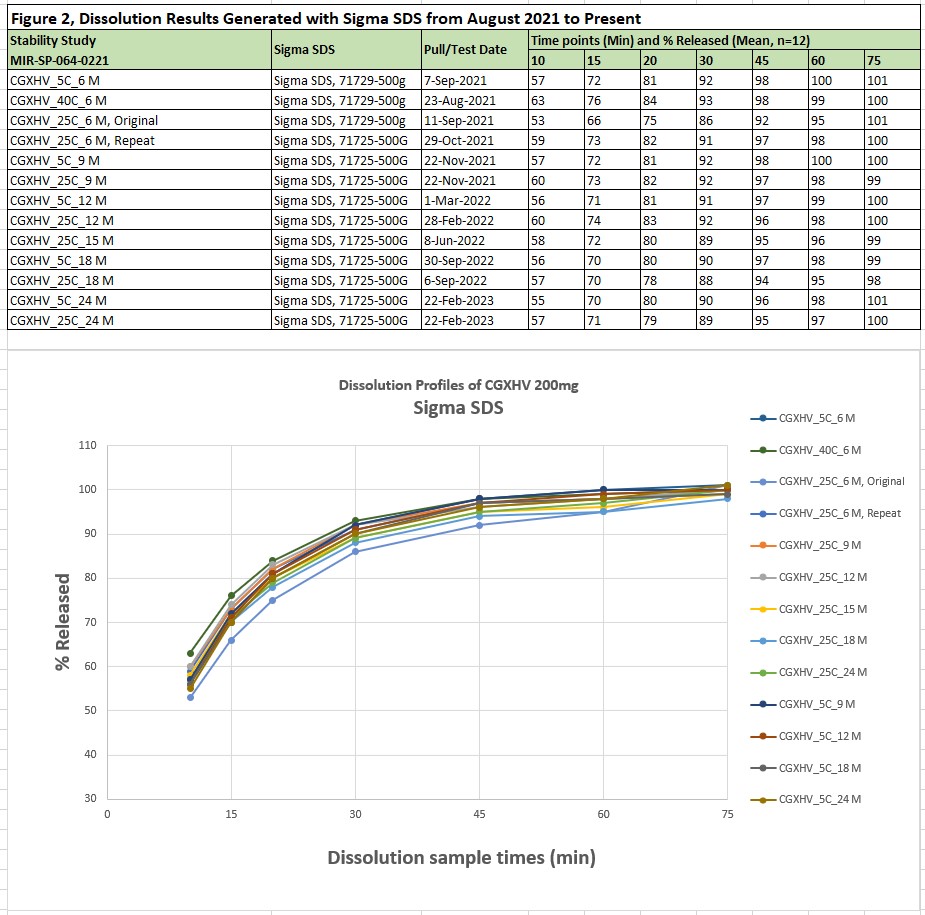 Figure 2, Dissolution Results Generated with Sigma SDS from August 2021 to Present
Figure 2, Dissolution Results Generated with Sigma SDS from August 2021 to Present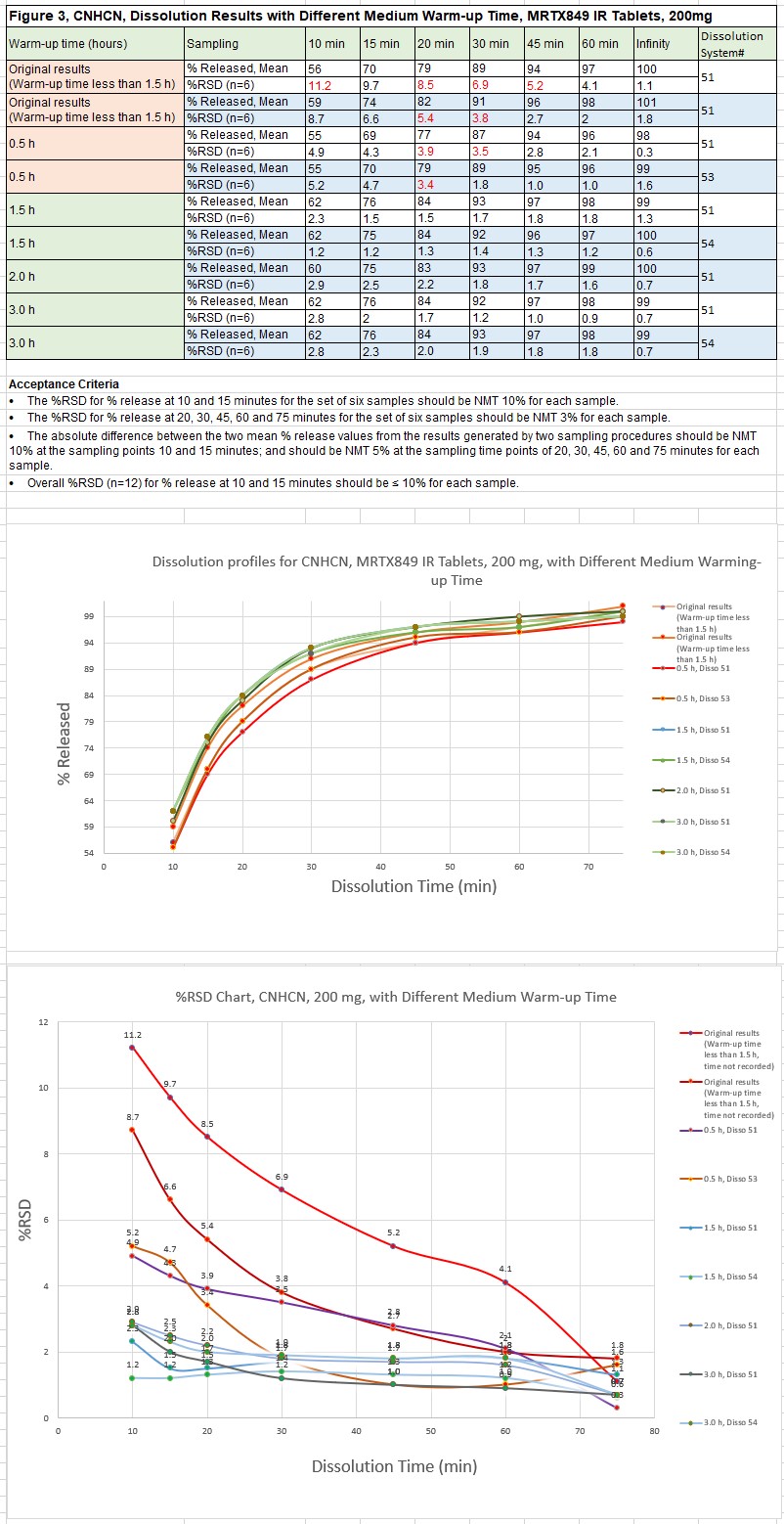 Figure 3, CNHCN, Dissolution Results with Different Warm-up Time, MRTX849 IR tablets, 200 mg
Figure 3, CNHCN, Dissolution Results with Different Warm-up Time, MRTX849 IR tablets, 200 mg