Preclinical and Translational Sciences - Biomolecular
Category: Poster Abstract
(T1230-10-65) A Survey of Prevalence of Pediatric Dosing for Biologics

Mohamed Ismail Nounou, PhD (he/him/his)
Clinical Pharmacology Reviewer
US Food and Drug Administration
Silver Spring, Maryland, United States
Mohamed Ismail Nounou, PhD (he/him/his)
Clinical Pharmacology Reviewer
US Food and Drug Administration
Silver Spring, Maryland, United States
Mohamed Ismail Nounou, PhD (he/him/his)
Clinical Pharmacology Reviewer
US Food and Drug Administration
Silver Spring, Maryland, United States- GB
Gilbert Burckart, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States .jpg)
Nada A. Helal, MS (she/her/hers)
PhD candidate
Texas A&M Health Sciences Center
College Station, Texas, United States- BE
Brianna Eales, MS (she/her/hers)
University of Houston
Houston, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: This study assessed the prevalence of pediatric dosing in novel ingredient biological drug products and evaluated the extent of population pharmacokinetics (PopPK) data usage to support dosing. The study aims to understand the tools used for dosing strategies, such as population pharmacokinetics (PopPK) analyses, pharmacokinetic (PK) modeling, modeling & simulation (M&S) and pharmacokinetics/pharmacodynamics (PK/PD) modeling that contribute to the derivation of pediatric dosing.
Methods: Based on products’ labelling from 2002 until 2021, 169 biologic drug products approved by the US Food and Drug Administration (FDA) were reviewed. Information collected from the label included: the biologic’s therapeutic protein/biologic class, inclusion of pediatric population, dosing regimen based on age groups, dosing strategy, the use of PopPK and the clinical trials conducted in pediatrics. PubMed, public FDA clinical pharmacology reviews and clinicaltrials.gov were used along with products’ FDA labels for data collection.
Results: A total of 169 biological products approved by the FDA between 2002 and 2021 were reviewed (Table 1). Based on the review conducted, 78 of the 169 (46.1%) products have an approved indication for pediatric use and contain dosing recommendations in the label. For the 78 products approved in pediatrics, there was a total of 149 clinical trials and/or utilization of PopPK used to support dosing. Phase 3 trials were the most commonly used in supporting pediatric dosing (47 of 78 products, 60.3%). PopPK use is comparable at 40 of 78 (51.3%) (Figure 1). PopPK and PK modelling supported the pediatric dosing by either helping to select (n=15) the dosing regimen or verify (n=25) the dosing that had already been studied (Figures 1 & 2).
Conclusion: PopPK analysis and modeling and simulation have long been applied to deriving pediatric dosing recommendations for small molecules and more recently, biological products. By understanding and learning from past cases on the use of pharmacometric tools to support pediatric dosing of biological products, methods can be further refined and optimized to ultimately increase the number of products available to children.
Acknowledgements: The opinions expressed in this article are those of the authors and should not be interpreted as the position of the US Food and Drug Administration.
.jpg) Table 1. Biologic marketed drugs from 2002 to 2021 noted in their chemical name. Bold names signify that they have pediatric dosing. This is a total of 169 drugs that were reviewed with 78 having pediatric dosing.
Table 1. Biologic marketed drugs from 2002 to 2021 noted in their chemical name. Bold names signify that they have pediatric dosing. This is a total of 169 drugs that were reviewed with 78 having pediatric dosing. 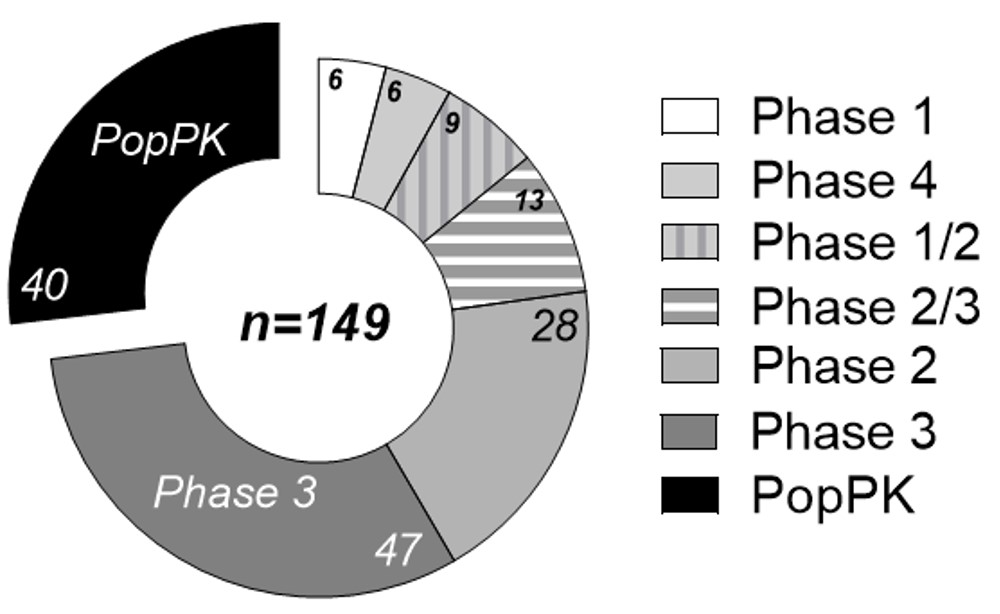 Figure 1. Clinical trials and PopPK of the 78 drugs approved for pediatrics between 2002 and 2021. 149 trials were conducted including pediatrics across the 78 biologic drugs that were approved for use in pediatrics.
Figure 1. Clinical trials and PopPK of the 78 drugs approved for pediatrics between 2002 and 2021. 149 trials were conducted including pediatrics across the 78 biologic drugs that were approved for use in pediatrics. 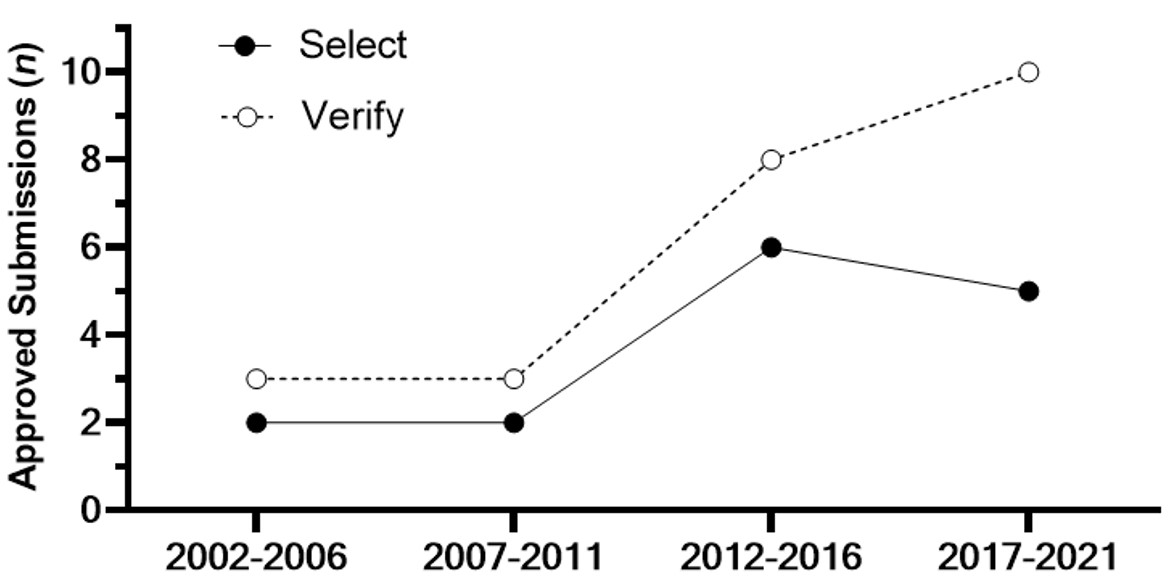 Figure 2. Trend of PopPK in five-year increments between 2002 and 2021 and the utilization to either select or verify pediatric dosing.
Figure 2. Trend of PopPK in five-year increments between 2002 and 2021 and the utilization to either select or verify pediatric dosing.