Formulation and Delivery - Chemical
Category: Poster Abstract
(T1430-11-75) Suitability of Isavuconazonium Sulfate as a Biopharmaceutics Classification System (BCS)-Based Biowaiver Candidate
Tuesday, October 24, 2023
2:30 PM - 3:30 PM ET

David Plano (he/him/his)
PhD student
Fraunhofer Institute for Translational Medicine and Pharmacology
Frankfurt Am Main, Hessen, Germany
David Plano (he/him/his)
PhD student
Fraunhofer Institute for Translational Medicine and Pharmacology
Frankfurt Am Main, Hessen, Germany
Niklas Rudolph (he/him/his)
PhD Student
Fraunhofer Institute for Translational Medicine and Pharmacology
Frankfurt am Main, Hessen, Germany- CS
Christoph Saal (he/him/his)
Boehringer Ingelheim
Biberach an der Riss, Baden-Wurttemberg, Germany - JD
Jennifer Dressman, Ph.D. (she/her/hers)
Fraunhofer Institute for Translational Medicine and Pharmacology
Frankfurt am Main, Hessen, Germany
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Generic drug products represent cost-effective alternatives to their originator counterparts while maintaining high quality and safety standards. A Biopharmaceutics Classification System (BCS) based biowaiver is a regulatory option to substitute suitable in vitro data for in vivo bioequivalence studies. Isavuconazonium sulfate is the prodrug of isavuconazole, an azole antifungal with broad spectrum and reduced toxicity. In this work we investigated the suitability of isavuconazonium sulfate as a candidate for the BCS biowaiver.
Methods: Following the ICH M9 guideline, solubility of isavuconazonium sulfate in USP dissolution media at pH 1.2, in acetate buffer at pH 4.5, and in phosphate buffer at pH 6.8 was measured using a downscaled shake-flask method [2]. Isavuconazonium sulfate drug substance was weighed into Whatman UniPrepTM chambers, and the respective degassed media was added to obtain a final concentration of 3 mg/mL [2,3]. Samples were incubated at 37°C and continuously shaken for 4 hours on an orbital shaker at 100 rpm. Samples were withdrawn after 5, 60, and 240 minutes. Applying the dose to solubility cutoff volume of 250 mL set by the ICH M9 guideline, isavuconazonium sulfate meets the BCS class I criterion for high solubility at a single dose of 186 mg. In vitro dissolution of the commercial marketed product Cresemba® (hard capsules) and the granulate (extracted from Cresemba® capsules) was tested in a USP type 2 dissolution apparatus at 50 rpm at 37°C with and without sinkers in 500 mL of each of the three media used for the solubility studies [1,3]. Samples were withdrawn at predefined time points, replacing the removed volume with fresh medium. All samples were filtered through 0.45 µm regenerated cellulose filters and immediately diluted with
40 mM phosphate buffer at pH 7.2. Samples were stored for 48 hours in an incubator at 40°C to convert isavuconazonium sulfate into isavuconazole for analytical reasons and subsequently analyzed via HPLC-UV.
All experiments were performed in triplicate.
Results: The solubility study confirmed the high literature solubility of isavuconazonium sulfate in aqueous media with values >1000 mg/mL in the range of pH 1.2-7.4 and 37°C (Figure 1 (Table 1)). When release from the commercial marketed capsule formulation was tested, 85 % or more release of isavuconazonium sulfate at pH 1.2 and 4.5 was attained only after 60 minutes due to slow disintegration of the hypromellose hard capsule. At pH 6.8, a maximum release of just 36% was measured after 50 minutes (Figure 2). An additional dissolution study was performed at pH 4.5 to investigate whether the use of sinkers is feasible. Results showed strong delay of capsule disintegration combined with a high standard deviation, confirming the poor performance of hypromellose as capsule material (Figure 3). Since the capsule shell disintegration was slow, release from the granulate without the capsule shell was also tested. The release was rapid and complete in all media (more than 85% within 5 minutes). While the concentration of isavuconazonium sulfate at pH 1.2 and 4.5 was stable, the concentration at pH 6.8 decreased by 40% over 2 hours, indicating hydrolysis to the parent drug (isavuconazole), which has a lower solubility and thus precipitates (Figure 2). Based on the ICH M9 guideline, a biowaiver is applicable if the commercial marketed dose is soluble in 250 mL or less in the physiological pH range of pH 1.2-6.8 and the formulation releases 85 % or more of the dose within 15 minutes [1,3]. Solubility studies indicated that isavuconazonium sulfate can be classified as “highly soluble”, even with the stability issue of isavuconazonium sulfate at pH 6.8 [1,3]. The poor disintegration behavior of the hypromellose capsule shell led to inconsistent and slow release profiles when performing dissolution tests with Cresemba®. Using sinkers exacerbated these effects. Nevertheless, isavuconazonium sulfate must be filled into hypromellose capsules because their lower water content (compared to hard gelatin capsules) prevents rapid conversion of the prodrug into isavuconazole. By contrast, the granulate showed complete and rapid release of more than 85% in less than 5 minutes, underlining that isavuconazonium sulfate is a highly soluble and rapidly dissolving drug substance. However, hydrolytic instability of the prodrug at pH 6.8 led to precipitation and decreasing concentrations of dissolved isavuconazonium over time.
Conclusion: Although isavuconazonium sulfate is a highly soluble drug, the commercial marketed capsule did not meet the criteria for a BCS-based biowaiver due to the slow and incomplete disintegration of the hypromellose capsule shell [1,3]. Since the dissolution criteria are not met by the reference listed product, it would not be possible to use the BCS based biowaiver for generic versions of isavuconazonium sulfate capsules.
References: [1] European Medicines Agency, 2020. ICH M9 guideline on biopharmaceutics classification system-based biowaivers, EMA/CHMP/ICH/493213/2018.
[2] A. Glomme, J. März, J.B. Dressman, Comparison of a miniaturized shake-flask solubility method with automated potentiometric acid/base titrations and calculated solubilities, J. Pharm. Sci. 94 (2005) 1–16. doi: 10.1002/jps.20212
[3] ICH, Biopharmaceutics Classification System-based Biowaivers: M9 (2019).
Acknowledgements: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this abstract.
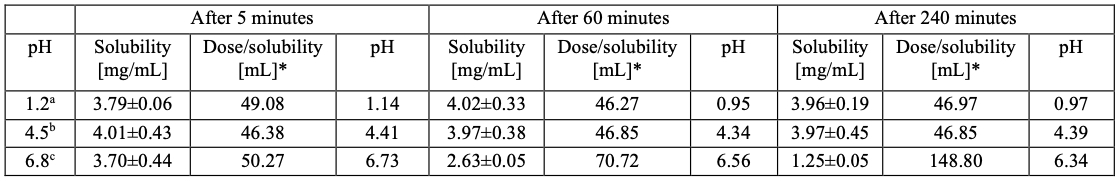 Figure 1: Experimentally determined solubility values for isavuconazonium sulfate at pH 1.2, 4.5 and 6.8 after 5, 60 and 240 minutes.
Figure 1: Experimentally determined solubility values for isavuconazonium sulfate at pH 1.2, 4.5 and 6.8 after 5, 60 and 240 minutes.a 0.1 N hydrochloride acid; b Acetate buffer; c Phosphate buffer
* Based on a single dose of 186 mg isavuconazonium sulfate (equivalent to 100 mg isavuconazole)
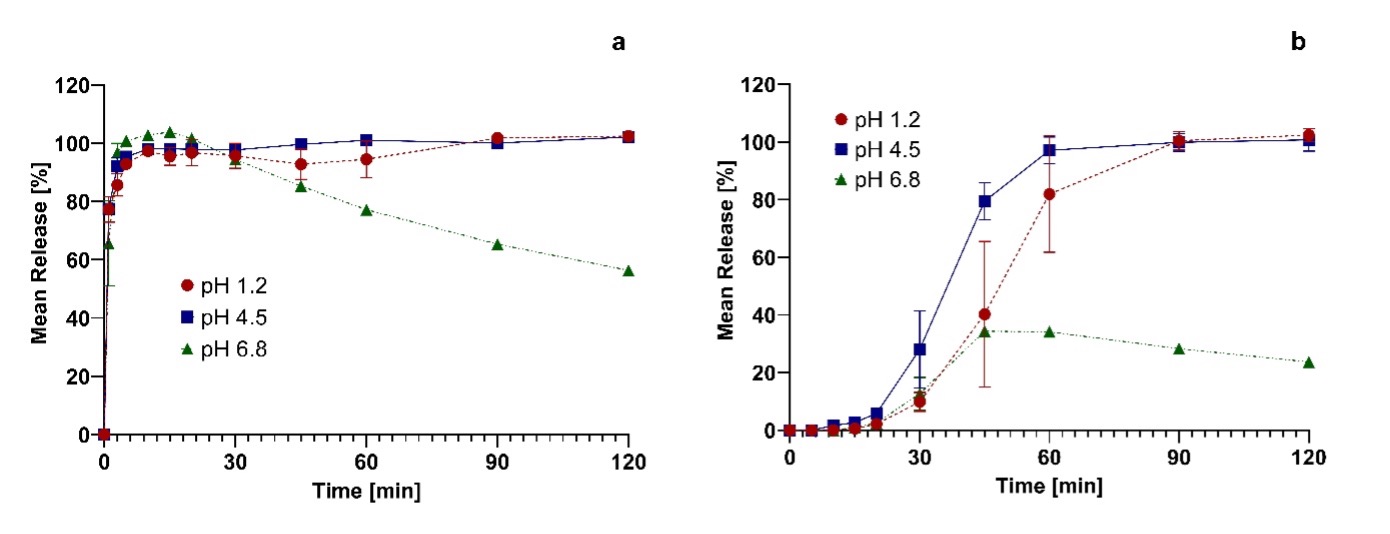 Figure 2: Mean release of Cresemba capsules and the extracted powder of Cresemba capsules in different compendial dissolution buffers (1.2, 4.5 and 6.8).
Figure 2: Mean release of Cresemba capsules and the extracted powder of Cresemba capsules in different compendial dissolution buffers (1.2, 4.5 and 6.8).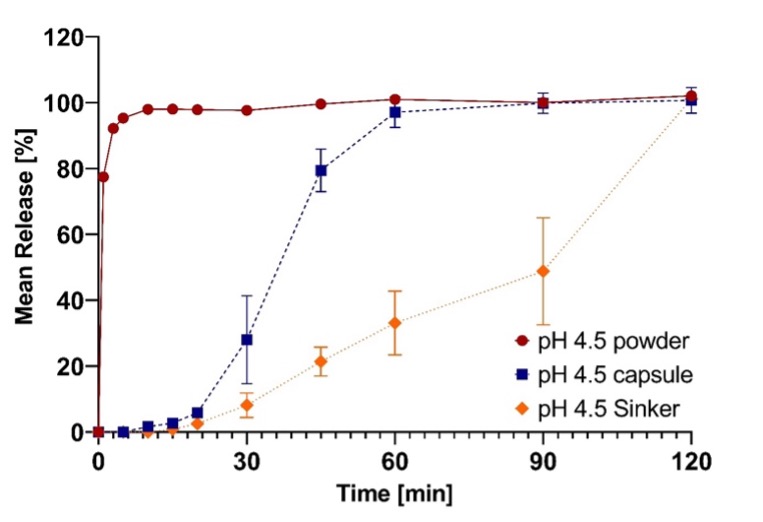 Figure 3: Comparison of the mean release of the extracted powder of Cresemba hard capsules and Cresemba hard capsules with and without sinkers in compendial dissolution acetate buffer at pH 4.5.
Figure 3: Comparison of the mean release of the extracted powder of Cresemba hard capsules and Cresemba hard capsules with and without sinkers in compendial dissolution acetate buffer at pH 4.5.