Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M1130-08-52) Enabling Formulation Development of LAIs Thorough Understanding Critical Formulation Parameters
- NM
Nilesh Malavia, M.S. (he/him/his)
University of Connecticut
Storrs, Connecticut, United States - NM
Nilesh Malavia, M.S. (he/him/his)
University of Connecticut
Storrs, Connecticut, United States - DA
Daniela Amaral Amaral Silva, Ph.D. (she/her/hers)
Scientist I
Simulations Plus, Inc.
Lancaster, California, United States - VL
Viera Lukacova, Ph.D.
Chief Scientist
Simulations Plus, Inc.
Lancaster, California, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - KA
Khondoker Alam, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States 
Diane J. Burgess, Ph.D. (she/her/hers)
Distinguished Professor
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: There has been tremendous growth in the development of long-acting injectable (LAI) suspensions in the past several years. These products consist of lipophilic active pharmaceutical ingredients dispersed in suspending media containing various inactive ingredients. Upon subcutaneous or intramuscular administration, a depot forms, and the drug is released slowly in a controlled manner. Despite an increase in the number of new drug applications approved by US Food and Drug Administration, there is a lack of generic equivalents of LAIs. This is probably due, at least in part, to the product complexity. Accordingly, there is an immediate need to understand the critical formulation parameters that may affect product performance in vitro and in vivo with a goal of developing in vitro-in vivo correlations (IVIVCs). However, the dissolution methods reported for these products are rapid, lasting up to two days, and thus may not be able to discriminate between LAI formulations and also are not sufficiently long to support IVIVC development [1]. Accordingly, the objective was to develop an in vitro release method with longer duration, which may have better correlation with in vivo drug release. A further objective was to characterize the reference listed drug (RLD) and Q1/Q2 formulations to understand the critical formulation parameters that could significantly impact product performance. This research may enable the development of LAI generic equivalent drug products.
Methods: To understand the behavior of LAIs, different Q1/Q2 formulations of medroxyprogesterone acetate (MPA) were prepared. Due to the limited formulation scope, particle size and polymer source variation were selected as critical formulation parameters. Formulations named as “FA” and “FC” were prepared using PEG3350 polymer from different sources. Antisolvent and top-down methods were utilized to change the drug particle size in formulations named as “FB”, “FD”, and “FE”. Solid-state characterization of MPA recrystallized via an antisolvent method was conducted using differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and particle size analysis by X-ray diffraction (PXRD). The formulations along with the RLD were then tested for drug content, solubility, viscosity, F-value [2], particle size, size distribution and particle morphology. To assess whether the suspensions were in the agglomerated state or not, particle size analysis was conducted in the presence of ultrasonic energy. Since the PEG3350 polymer is sensitive to shear, gel permeation chromatography was performed to determine the molecular weight after processing. In order to understand the drug release and to extend the release duration, a novel adapter was designed for LAIs release testing using a USP type IV apparatus. The data generated using the novel adapter were compared with data obtained using the traditional semisolid adapters [3].
Results: The formulations were consistent in their targeted drug concentration with good content uniformity. The MPA formulation generated via an antisolvent method (formulation FB) was crystalline and free of residual solvent (PXRD and thermal analysis). Particle size analysis (Malvern Mastersizer and microscopy) revealed that formulation FB had the largest particle size. The particle morphology of the RLD, FA, and FC were similar. However, any alteration of MPA via recrystallization, homogenization or milling modified particle morphology, which affected surface area and packing. In addition, changing drug particle size affected suspension aggregation, as verified by particle size analysis. Interestingly, formulations FA, FC, and the RLD experienced a decrease in particle size on application of ultrasonic energy, whereas formulations FB, FD, and FE remained constant. This implies that formulations FA, FC, and RLD were in a state of flocculation, while formulations FB, FD, and FE were deflocculated. Formulations FB has large particle size and therefore is likely to undergo rapid sedimentation in the deflocculated state. Formulations FD and FE have small particle size leading to large surface area and accordingly there may be insufficient polymer in these formulations to maintain flocculation. There were significant differences in the release behavior of formulations with varying particle sizes. However, the excipient source did not affect drug release. The formulation with the largest particle size, FB, showed the slowest release, which was attributed to decreased surface area for dissolution. Formulations FD and FE had similar particle sizes but showed different release behavior. For FD formulation, the release behavior was attributed to closer particle packing, resulting from their fractured irregular shapes, which effectively reduces the surface area for dissolution. Whereas the FE formulation particles had smooth spherical surfaces which are not conducive to close packing. The novel adapter method achieved relatively long drug release profiles and was able to discriminate among formulations. Whereas, the semisolid adapter method was not able to achieve long release duration and showed unacceptable error.
Conclusion: The performance of LAI drug products appears to be influenced by particle size, surface morphology and the degree of flocculation. The developed novel adapter method showed good discriminatory ability and reproducibility, as well as a relatively long release duration.
References: [1] Bao, Q., Wang, X., Wan, B., Zou, Y., Wang, Y., & Burgess, D. J. (2023). Development of in vitro-in vivo correlations for long-acting injectable suspensions. International Journal of Pharmaceutics, 634, 122642.
[2] Tempio, Joseph S., and Joel L. Zatz. "Flocculation effect of xanthan gum in pharmaceutical suspensions." Journal of Pharmaceutical Sciences 69, no. 10 (1980): 1209-1214.
[3] Bao, Q., Wang, X., Zou, Y., Wang, Y., & Burgess, D. J. (2022). In vitro release testing method development for long-acting injectable suspensions. International Journal of Pharmaceutics, 622, 121840.
Acknowledgements: The project was funded by a contract from the U.S. Food and Drug Administration (contract # 75F40121C00133). The views expressed here do not reflect official policies of the US FDA or the Department of Health and Human Services, nor does any mention of trade names imply endorsement by the US Government.
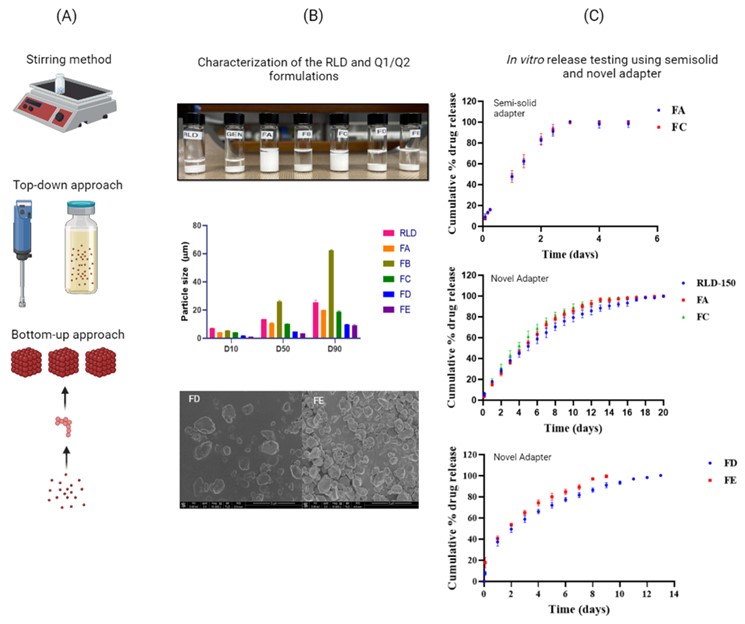 Fig. 1. Schematic representation of: (A) Preparation of Q1/Q2 formulations via simple stirring method, top-down and bottom-up approaches; (B) Physicochemical characterization of the RLD and different Q1/Q2 formulations; and (C) In vitro drug release profiles of the RLD and Q1/Q2 formulations generated by using semi-solid and novel adapter in USP type IV apparatus. Data are expressed as mean ± SD (n=3)
Fig. 1. Schematic representation of: (A) Preparation of Q1/Q2 formulations via simple stirring method, top-down and bottom-up approaches; (B) Physicochemical characterization of the RLD and different Q1/Q2 formulations; and (C) In vitro drug release profiles of the RLD and Q1/Q2 formulations generated by using semi-solid and novel adapter in USP type IV apparatus. Data are expressed as mean ± SD (n=3)