Formulation and Delivery - Biomolecular
Category: Poster Abstract
(M1030-01-01) Breaking the Pain Barrier: Innovative Strategies for Administering a Whole Cell Inactivated Microparticulate Gonorrhea Vaccine
Monday, October 23, 2023
10:30 AM - 11:30 AM ET
- PB
Priyal Bagwe, BS (she/her/hers)
Mercer University
Atlanta, Georgia, United States - PB
Priyal Bagwe, BS (she/her/hers)
Mercer University
Atlanta, Georgia, United States - SS
Sarthak Shah, MS (he/him/his)
PhD Student
Mercer University
atlanta, Georgia, United States - SZ
Susu Zughaier, Ph.D. (she/her/hers)
Qatar University
Doha, Ad Dawhah, Qatar - MD
Martin J. D'Souza, Ph.D.
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Gonorrhea is a sexually transmitted mucosal disease caused by the bacterium Neisseria gonorrhoeae, which primarily infects the mucosal tissues. Due to the rise in antimicrobial resistance, the public health threat posed by Neisseria gonorrhoeae is significant. To date, there are no vaccines approved by the FDA for preventing gonorrhea. Current gonorrhea vaccines in clinical trials are administered via the intramuscular or subcutaneous route, except for one recent vaccine candidate endorsed for intranasal vaccination. The intramuscular or subcutaneous routes are invasive and require skilled personnel for administration. We have developed a whole-cell inactivated microparticulate vaccine containing adjuvants for gonorrhea. This vaccine candidate generated a robust immune response when administered by non-invasive microneedles via the transdermal route. Moreover, since gonorrhea is a mucosal disease, the buccal and nasal mucosa are also excellent sites for vaccine administration due to the rich vasculature and lymphatic drainage systems consisting of resident immune cells, dendritic cells, and M-cell-like-cells, which can actively engulf the vaccine antigen and present it to T Cells. In this regard, we aimed to explore different vaccine delivery routes by comparing the antibody responses, cellular responses, immune correlates of protection, and resistance to infection generated upon intranasal, buccal, transdermal, and intramuscular vaccination.
Methods: Neisseria gonorrhoeae strain CDC-F62 was grown and inactivated with formalin. This whole-cell inactivated gonorrhea was encapsulated into a biodegradable pre-crosslinked albumin matrix. Gonococci vaccine microparticles (Gc-MP) and adjuvant MP (Alum and AddaVax™) were prepared by a method previously developed in our laboratory using the Buchi Mini Spray Dryer B-290. The in vivo efficacy of GC-MP with adjuvant MP was assessed using female Swiss Webster mice immunized with one prime and two booster doses on days 0, 14, and 28. For transdermal vaccination, we used dissolving microneedles as the dosage form. The dorsal area of the mice was shaved using depilatory cream one day before immunization. The microneedle was applied to the shaved area for 10 minutes until the needles dissolved. For intranasal administration, the adjuvanted vaccine microparticles were suspended in normal saline and delivered as 20 µL/ nostril nasal drops. For buccal administration, the orally dissolving films (ODFs) were placed on the buccal cheek area, followed by which the mice were fasted for two hours. For intramuscular administration, the adjuvanted vaccine microparticles were suspended in 100 μL of phosphate-buffered saline solution and injected. Subsequently, serum samples were collected every alternate week to measure antigen-specific IgG, IgM, IgG1, IgG2a, and IgA levels using enzyme-linked immunosorbent assay (ELISA). Additionally, the immunized mice sera were also assessed for its bactericidal activity. Post-vaccination, at week 8, the mice were challenged with live Neisseria gonorrhoeae strain, CDC-F62, using a murine lower genital tract infection model at 106 CFU/mL. At the end of the study, the mice were sacrificed. Immune organs such as spleens and lymph nodes were harvested to assess the expression of CD4 and CD8 using flow cytometry.
Results: Gonococci MP and adjuvant MP were successfully formulated and loaded in the microneedles, IN drops, and buccal ODFs for administration. ELISA demonstrated significantly higher levels of IgM which underwent isotype switching to IgG after week 2 in groups receiving the adjuvanted gonorrhea vaccine via IM, MN, IN, and ODFs. The group receiving MN (p < 0.0001) had significantly higher IgG when compared to other groups, but all the groups had relatively higher levels compared to no treatment group. IgG1 and IgG2a in all the vaccine groups were higher when compared to the control group (p < 0.0001). The antibodies generated in mice after immunization via IM (p < 0.05), MN (p < 0.05), IN (p < 0.001), and ODF (p < 0.001) routes were bactericidal towards the gonorrhea antigen at 1:1000 dilution titer compared to the control group. The mice who received the vaccine via IM and MN cleared the infection in 6-9 days, IN in 7-9 days, ODFs in 8-10 days, and the control group in 10-12 days. IgA levels were significantly higher for the groups that received the vaccine via the mucosal routes, i.e., IN and ODF (p < 0.0001). The mice that received the gonococcal vaccine via all routes expressed significantly higher CD4 (p < 0.05) and CD8 (p < 0.0001) levels when compared to the control group in the spleen and lymph nodes.
Conclusion: This study explores different pain-free routes for delivering a potential vaccine for gonorrhea. We compared the in vivo immunogenicity, correlates of protection, and resistance to infection of our novel gonorrhea vaccine. All the routes generated strong immunity. Moreover, the transdermal route generated a significantly more robust systemic immune response when compared to all other vaccine routes—the intranasal and buccal mucosal routes generated significantly higher mucosal immunity. The immune responses induced by the pain-free strategies were comparable to the conventional intramuscular route. Our findings indicate the route of administration of the gonococcal microparticulate vaccine affects the type of immune responses generated.
References: Gala RP, Zaman RU, D'Souza MJ, Zughaier SM. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018;6:60. https://doi.org/10.3390/vaccines6030060.
Bagwe P, Bajaj L, Gala RP, D’Souza MJ, Zughaier SM. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022;10:983. https://doi.org/10.3390/vaccines10070983.
Menon I, Bagwe P, Gomes KB, Bajaj L, Gala R, Uddin MN, et al. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021;12:435. https://doi.org/10.3390/mi12040435.
Acknowledgements: The project is funded by an NIH grant 1R15AI133473-01A1.
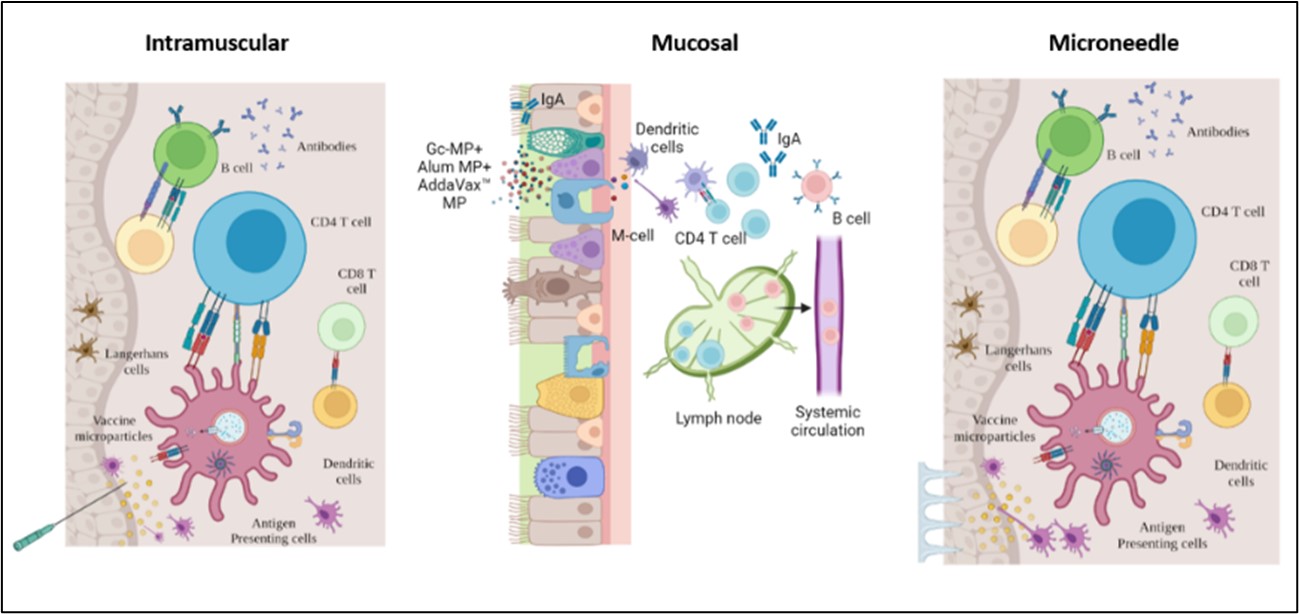 Figure 1. Exploring pain-free vaccination alternatives for a whole cell inactivated microparticulate gonorrhea vaccine. The mucosal immune system consists of specialized cells, including T and B lymphocytes, antigen-presenting cells, and secretory IgA antibodies. The primary function of mucosal immunity is to provide protection against pathogens that enter the body through mucosal surfaces. Unlike the systemic immune response, which mainly produces IgG antibodies, mucosal immunity produces IgA antibodies, which are secreted into the mucosal secretions and can neutralize pathogens before they can establish an infection. Transdermal immunization using dissolving microneedles is another attractive pain-free alternative. The skin contains specialized antigen-presenting cells, including Langerhans cells, dermal dendritic cells, and resident macrophages, making it a promising site for immunization. Langerhans cells play a crucial role in immune surveillance and signaling to T cells, which are drained into nearby lymph nodes, stimulating B cells to produce antibodies.
Figure 1. Exploring pain-free vaccination alternatives for a whole cell inactivated microparticulate gonorrhea vaccine. The mucosal immune system consists of specialized cells, including T and B lymphocytes, antigen-presenting cells, and secretory IgA antibodies. The primary function of mucosal immunity is to provide protection against pathogens that enter the body through mucosal surfaces. Unlike the systemic immune response, which mainly produces IgG antibodies, mucosal immunity produces IgA antibodies, which are secreted into the mucosal secretions and can neutralize pathogens before they can establish an infection. Transdermal immunization using dissolving microneedles is another attractive pain-free alternative. The skin contains specialized antigen-presenting cells, including Langerhans cells, dermal dendritic cells, and resident macrophages, making it a promising site for immunization. Langerhans cells play a crucial role in immune surveillance and signaling to T cells, which are drained into nearby lymph nodes, stimulating B cells to produce antibodies.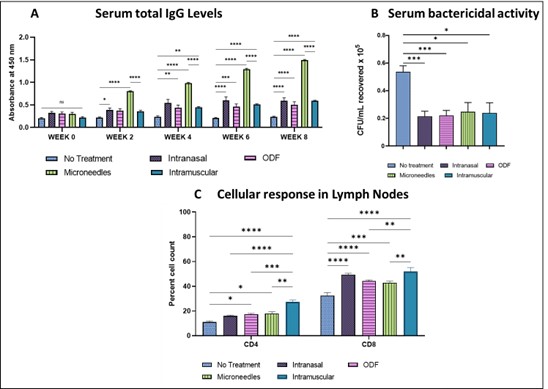 Figure 2. A. Microneedle, intranasal, buccal, and intramuscular immunization with whole-cell inactivated gonococci vaccine MP generated antibodies against N. gonorrhoeae. The mice were immunized with three doses of Gc-MP (100 µg) combined with adjuvants (Alum MP or AddaVax™ MP). Dilution Titer- 1:100. B. Serum Bactericidal Assay. Colony Forming Units recovered (CFU/mL) of N. gonorrhoeae exposed to mice sera at 1:1000 dilution. Data are expressed as mean ± SEM, N=5 mice, Two-way repeated measures ANOVA, * p < 0.05 significant, ** p < 0.01 very significant, *** p < 0.001 extremely significant, **** p < 0.0001 extremely significant. C. Expression of CD4 and CD8 on the surface of the lymph node cells from vaccinated mice. The mice were sacrificed two weeks after the challenge study's beginning. The surface expression of the CD4 Lymph node and CD8 Lymph node induced by MN, ODF, IN, and IM immunization was compared to no treatment control cells. Data are expressed as Mean ± S.E.M., N=5 mice, *** p < 0.001 extremely significant, ** p < 0.01; * p < 0.05 (Brown Forsythe ANOVA test).
Figure 2. A. Microneedle, intranasal, buccal, and intramuscular immunization with whole-cell inactivated gonococci vaccine MP generated antibodies against N. gonorrhoeae. The mice were immunized with three doses of Gc-MP (100 µg) combined with adjuvants (Alum MP or AddaVax™ MP). Dilution Titer- 1:100. B. Serum Bactericidal Assay. Colony Forming Units recovered (CFU/mL) of N. gonorrhoeae exposed to mice sera at 1:1000 dilution. Data are expressed as mean ± SEM, N=5 mice, Two-way repeated measures ANOVA, * p < 0.05 significant, ** p < 0.01 very significant, *** p < 0.001 extremely significant, **** p < 0.0001 extremely significant. C. Expression of CD4 and CD8 on the surface of the lymph node cells from vaccinated mice. The mice were sacrificed two weeks after the challenge study's beginning. The surface expression of the CD4 Lymph node and CD8 Lymph node induced by MN, ODF, IN, and IM immunization was compared to no treatment control cells. Data are expressed as Mean ± S.E.M., N=5 mice, *** p < 0.001 extremely significant, ** p < 0.01; * p < 0.05 (Brown Forsythe ANOVA test).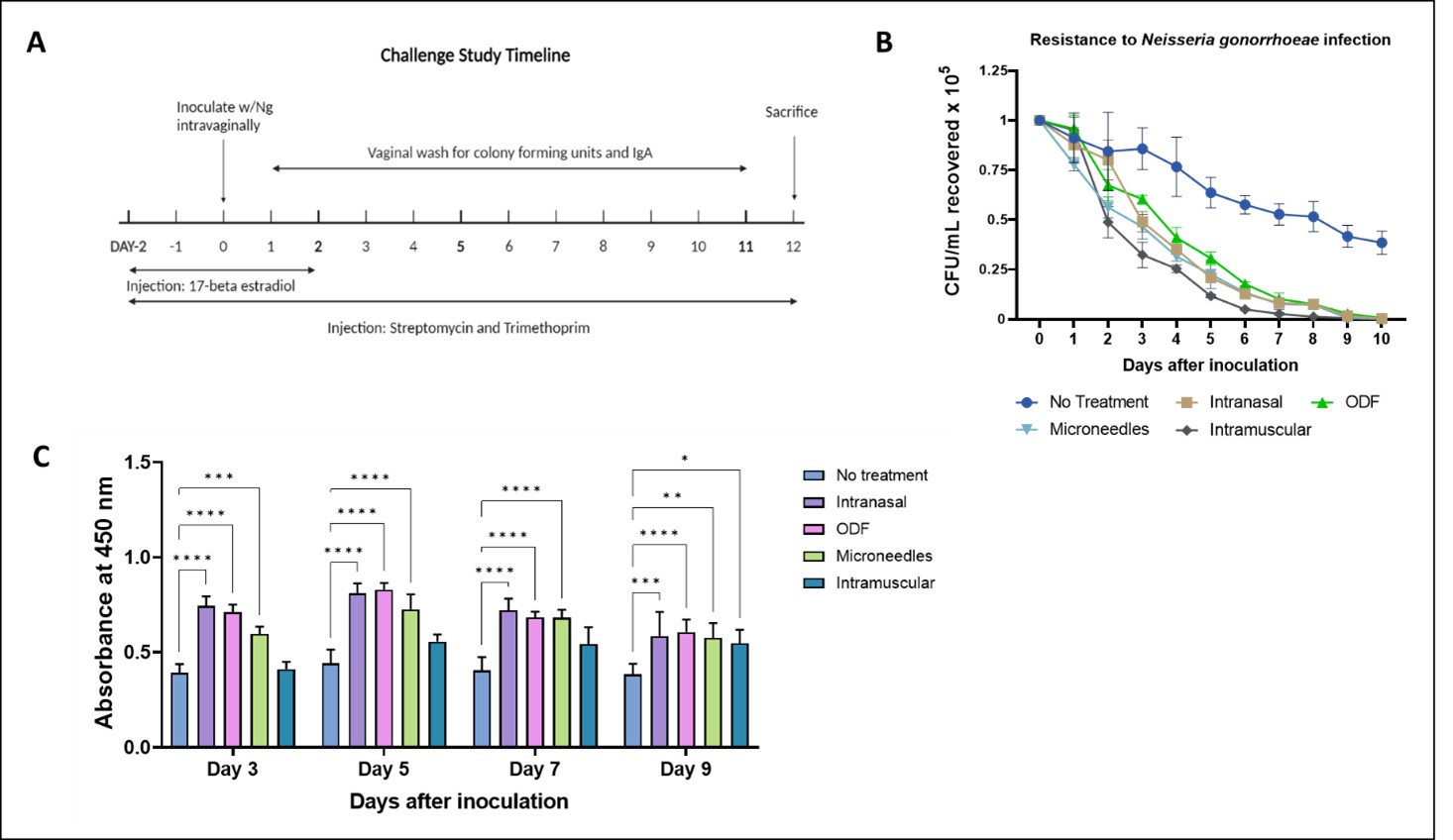 Figure 3. (A) Challenge Study Timeline: The mice were challenged at week 8 intra-vaginally with a live Neisseria gonorrhoeae strain, CDC-F62, in the murine lower genital tract infection model at 106 CFU/mL. The infection was monitored by analyzing vaginal washes cultured on agar plates. (B) Resistance to live bacterial infection in mice following challenge, CFU/mL recovered of N. gonorrhoeae from the mice immunized with the vaccine in MN, ODF, IN, and IM routes were compared to the control group. (C) Local vaginal IgA Levels (Absorbance at 450nm). Data are expressed as mean ± SEM, N=5 mice, **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 (ANOVA, no treatment vs. IM, IN, ODF, MN).
Figure 3. (A) Challenge Study Timeline: The mice were challenged at week 8 intra-vaginally with a live Neisseria gonorrhoeae strain, CDC-F62, in the murine lower genital tract infection model at 106 CFU/mL. The infection was monitored by analyzing vaginal washes cultured on agar plates. (B) Resistance to live bacterial infection in mice following challenge, CFU/mL recovered of N. gonorrhoeae from the mice immunized with the vaccine in MN, ODF, IN, and IM routes were compared to the control group. (C) Local vaginal IgA Levels (Absorbance at 450nm). Data are expressed as mean ± SEM, N=5 mice, **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05 (ANOVA, no treatment vs. IM, IN, ODF, MN).