Formulation and Delivery - Biomolecular
Category: Poster Abstract
(T1230-01-02) Immunity for the Long Haul: Exploring Cross-Protectivity against Emerging Strains upon Vaccination with Gonococcal Vaccine Microparticles in Mice
Tuesday, October 24, 2023
12:30 PM - 1:30 PM ET
- PB
Priyal Bagwe, BS (she/her/hers)
Mercer University
Atlanta, Georgia, United States - PB
Priyal Bagwe, BS (she/her/hers)
Mercer University
Atlanta, Georgia, United States - AF
Amarae Ferguson, BS (he/him/his)
Mercer University
Atlanta, Georgia, United States - SZ
Susu Zughaier, Ph.D. (she/her/hers)
Qatar University
Doha, Ad Dawhah, Qatar - MD
Martin J. D'Souza, Ph.D.
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: There is a global rise in the number of cases of gonorrhea infection each year. Neisseria gonorrhoeae causes gonorrhea and is currently being treated using antibiotics. Currently, there is no vaccine for gonorrhea. We have previously shown that a transdermal polymeric microneedle-based microparticulate vaccine with adjuvants allows for better uptake of antigen facilitated by the antigen-presenting cells (APCs), caused by improved antigen presentation and subsequent systemic immune response by activation of T cells. However, the continued emergence of antimicrobial resistance to gonorrhea necessitates testing the cross-protectiveness of our vaccine formulation. In this regard, our aim was 1) to assess the vaccine-induced cross-protection against a heterologous emerging drug-resistant gonorrhea strain, 2) to perform a dose-response analysis to understand the minimum dose required to generate a protective immune response and the maximum immune response generated, and as reinfection cases are common in gonorrhea due to the lack of a strong memory response 3) to assess the durability of the immune responses by re-infecting the mice with live gonorrhea.
Methods: N. gonorrhoeae strain CDC-F62 was grown and formalin-inactivated. This whole-cell inactivated gonorrhea was encapsulated into a biodegradable pre-crosslinked albumin matrix. Gonococci vaccine MP (Gc-MP) and adjuvant MP (Alum and AddaVax™) were prepared by a method previously developed in our laboratory using the Buchi Mini Spray Dryer B-290. The MP with 20% antigen loading was mixed with hyaluronic acid and trehalose to form an aqueous solution. This solution was then loaded into the MN molds (10x10 array), and the MN was created using the spin casting method. The mice were divided into groups receiving 50, 100, and 200 µg of antigen dose. The adjuvant amount was 50 µg each of Alum and MF59 for all the groups. The mice were given one prime and two booster doses in weeks 0, 2, and 4. The induction of antibodies was measured using ELISA to detect the levels of IgG, its sub-types, and IgM in the collected mice sera. The ability of the adjuvanted gonococci vaccine MP to confer cross-protection in vivo was assessed. Following vaccination, at week 10, the mice were challenged with live Neisseria gonorrhoeae strains, CDC-F62 and FA1090, at 106 CFU/mL. The groups were divided into two subgroups where one was challenged with the parent strain CDC-F62, and the other was challenged with the heterologous strain FA1090. Gonorrhea growth pattern was monitored in the vaginal washes during the challenge. To evaluate the duration of these induced immunity, we re-infected the mice four weeks after the first challenge and analyzed the resistance to reinfection. Also, two weeks after the challenge, we sacrificed the mice. The primary and secondary immune organs were harvested to assess the cellular immune response, CD4, CD8 expression, and long-term memory response, CD62, CD45 using flow cytometry.
Results: Adjuvanted gonococci MP were successfully formulated and loaded in the microneedles in 50 µg, 100 µg, and 200 µg antigen doses. The immune response generated was dose-dependent. ELISA demonstrated that the mice receiving 200 µg antigen dose had significantly higher levels of IgG, IgG1, and IG2a compared to other treatment groups and control from week 2 through week 8. IgM levels peaked at week 2 for all the vaccine groups, but the isotype switched to IgG after the prime dose. For the challenge study with the parent strain, the mice that received the 200 µg & 100 µg doses cleared the first infection in 6-9 days and the reinfection in 4-7 days. These groups cleared the infection relatively faster when compared to other treatment groups and control groups. For the challenge study with the heterologous strain, the mice that received the 200 µg & 100 µg doses cleared the first infection in 7-9 days and the reinfection in 6-9 days. The mice that received the 200 µg & 100 µg doses showed a higher CD4 expression in cells from the spleen (p < 0.001) & lymph nodes (p < 0.05) and CD8 expression in cells from lymph nodes (p < 0.001). All the mice groups that received the vaccine doses had a higher fraction of CD62L and CD45 memory markers expression in spleen cells. The mice group receiving 200 µg dose had higher CD62L (p < 0.05) and CD45 (p < 0.01) expression in lymph nodes.
Conclusion: This study explores the different doses, cross-protectivity, and memory response for a potential vaccine for gonorrhea. We compared the dose-response strategy, in vivo immunogenicity, correlates of protection, resistance to infection, reinfection, and memory response of our novel gonorrhea vaccine. 100 µg and 200 µg doses generated strong immunity. Moreover, there was no significant difference between the immune responses of the two doses. There was cross-protection against a heterologous strain. Additionally, the vaccine generated a memory response. Our findings indicate longevity and cross-protection upon vaccination with our gonorrhea vaccine candidate.
References: Gala RP, Zaman RU, D’Souza MJ, Zughaier SM. Novel Whole-Cell Inactivated Neisseria Gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018;6:60. https://doi.org/10.3390/vaccines6030060.
Menon I, Bagwe P, Gomes KB, Bajaj L, Gala R, Uddin MN, et al. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021;12:435. https://doi.org/10.3390/mi12040435.
Liu, Y., Hammer, L., Liu, W. et al. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol 10, 1594–1608 (2017). https://doi.org/10.1038/mi.2017.11
Acknowledgements: The project is funded by an NIH grant 1R15AI133473-01A1.
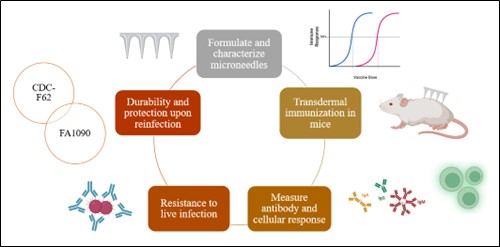 Figure 1. Study design- Immune for the long haul: Exploring cross-protectivity against emerging strains upon vaccination with gonococcal vaccine microparticles in mice.
Figure 1. Study design- Immune for the long haul: Exploring cross-protectivity against emerging strains upon vaccination with gonococcal vaccine microparticles in mice.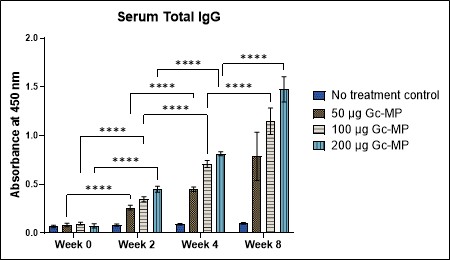 Figure 2. Serum Total IgG Levels- Microneedle immunization with whole-cell inactivated gonococci vaccine MP generated antibodies against N. gonorrhoeae. The mice were immunized with three doses of Gc-MP (50, 100, and 200 µg) combined with adjuvants (Alum MP and MF59 MP). Dilution Titer- 1:100. Data are expressed as mean ± SEM, N=5 mice, Two-way repeated measures ANOVA, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 2. Serum Total IgG Levels- Microneedle immunization with whole-cell inactivated gonococci vaccine MP generated antibodies against N. gonorrhoeae. The mice were immunized with three doses of Gc-MP (50, 100, and 200 µg) combined with adjuvants (Alum MP and MF59 MP). Dilution Titer- 1:100. Data are expressed as mean ± SEM, N=5 mice, Two-way repeated measures ANOVA, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. .jpg) Figure 3. Left panel- Expression of CD4 T cells on the surface of the spleen cells; Middle panel- Expression of CD4 T cells on the surface of the lymph node cells; and Right panel- Expression of CD8 T cells on the lymph node cells. The mice were sacrificed two weeks after the second reinfection challenge. The surface expression of CD4 Spleen, CD4 Lymph node, and CD8 Lymph node induced by different vaccine groups was compared to no treatment control cells. Data are expressed as Mean ± S.E.M., N=5 mice, *** p < 0.001, ** p < 0.01; * p < 0.05 (Brown Forsythe ANOVA test).
Figure 3. Left panel- Expression of CD4 T cells on the surface of the spleen cells; Middle panel- Expression of CD4 T cells on the surface of the lymph node cells; and Right panel- Expression of CD8 T cells on the lymph node cells. The mice were sacrificed two weeks after the second reinfection challenge. The surface expression of CD4 Spleen, CD4 Lymph node, and CD8 Lymph node induced by different vaccine groups was compared to no treatment control cells. Data are expressed as Mean ± S.E.M., N=5 mice, *** p < 0.001, ** p < 0.01; * p < 0.05 (Brown Forsythe ANOVA test).