Formulation and Delivery - Chemical
Category: Poster Abstract
(M1230-02-08) A Quality-by-Design Approach for the Development of Inhalable Dry Powder Containing Dual Drug-Loaded Polymeric Nanoparticles for Pulmonary Tuberculosis Treatment
Monday, October 23, 2023
12:30 PM - 1:30 PM ET

Suyash M. Patil, B.S.
PhD Student
St. John's University
Jamaica, New York, United States
Suyash M. Patil, B.S.
PhD Student
St. John's University
Jamaica, New York, United States- AD
Alec M. Diorio, B.S. (he/him/his)
St. John's University
New York, New York, United States - PK
Parasharamulu Kommarajula, M.S. (he/him/his)
St. John's University
flushing, New York, United States - NK
Nitesh Kunda, Ph.D.
St. John's University
New York, New York, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Tuberculosis (TB) is caused by the bacillus Mycobacterium tuberculosis (M.tb) and is the second leading cause of death from an infectious disease globally. The disease mainly affects the lungs and forms granulomatous lesions that encapsulate the bacteria, making treating TB challenging. The current treatment includes oral administration of bedaquiline (BDQ) and pretomanid (PTD); however, patients suffer from severe systemic toxicities, low lung drug concentration, and non-adherence. Therefore, we propose formulating BDQ-PTD drug combination co-loaded into polymeric nanoparticles and administered as dry powder for inhalation. The combination therapy will avoid the development of resistance, and inhalation delivery will ensure local drug deposition and patient compliance by lowering systemic exposure and toxicities. Toward this, Quality-by-Design (QbD), a risk-based approach, was implemented to maximize the use of resources and develop efficient design space based on prior knowledge of spray drying.
Methods: The effect of a dual-drug (BDQ and PTD) combination in M.tb bacteria (H37Ra, ATCC 25177TM) was confirmed by computing minimum inhibitory concentration (MIC) using resazurin microtiter assay (REMA). The nanoprecipitation method was used to prepare PLGA (poly(lactic-co-glycolic acid)) nanoparticles containing optimized drug ratio (1:4 – BDQ:PTD). As part of QbD, Risk assessment (RA), was performed based on previous knowledge and literature. Firstly, the quality target product profile (QTPPs) was identified to obtain desired product properties, and the factors influencing QTPPs, i.e., critical quality attributes (CQAs) and critical process parameters (CPPs), were determined for the spray drying preparation method. Secondly, the interaction rating between QTPPs, CQAs, and CPPs was used to rank the influence of each CQA and CPP on the final product. Finally, the three-factorial and three-level Box-Behnken Design was used to assess the effect of CPPs on product quality (CQAs) by DesignExpert® software. A total of 15 runs were performed and the obtained dry powders were characterized for outlet temperature, yield, moisture content, particle size after reconstitution, and aerosolization performance. The Next generation impactor (NGI™ M-170) was used to assess aerosolization performance, Zetasizer (Malvern Instruments) to measure nanoparticle size, and thermogravimetric analysis to measure moisture content.
Results: The MIC of BDQ and PTD in M.tb was 0.063µg/mL (Fig.1A) and 0.250µg/mL (Fig.1B), respectively. However, the BDQ-PTD combination showed a 2-fold reduction in MIC values (BDQ 0.016 µg/mL and PTD 0.063 µg/mL; Fig.1C)) compared to individual drugs. In addition, 1:4 BDQ:PTD ratio showed an additive effect with an FIC index of 0.5 (Fig.1D). Further, we successfully loaded optimized drug ratio in PLGA nanoparticles with 82.0±5.4% and 92.5±4.6% encapsulation efficiency for BDQ and PTD, respectively. Based on the knowledge space for spray drying, we identified and reported the QTPPs, CQAs, and CPPs to achieve desired product quality (Fig.2A). The interdependence rating, highlighted by low, medium, and high, shows the interaction of CQAs with QTPPs (Fig.2B) and CPPs (Fig.2C). Further, Pareto charts were generated and show the ranking of the CQAs (Fig.2D) and CPPs (Fig.2E) based on the severity score for each parameter. Based on RA, the target quality of the product is primarily dependent on aerosolization performance (MMAD and FPF) followed by nanoparticle size, drug loading, moisture content, and dry powder yield (Fig.2D). Further, these CQAs are then achieved by optimizing the CPPs which are primarily dependent on outlet temperature followed by spray-dryer parameters of inlet temperature, feed rate, and aspirator and are least influenced by the excipients-to-NPs ratio (Fig.2E). Hence, design space was prepared by setting the process parameters: drying/inlet temperature (X1; 80-120ºC), aspirator (X2; 80-100%), and feed rate (X3; 2-6mL/min) as input variables, and each factor was divided into three levels. The responses of the dry powders prepared using Büchi B-290 mini spray dryer (1:1.5 for NPs:L-leucine suspension) were outlet temperature (R1), powder yield (R2), mass median aerodynamic diameter (MMAD; R3), fine particle fraction (FPF; R4), and nanoparticle size (R5). The Design of Experiments (DoE) analysis showed a linear model for outlet temperature (Fig.3A), a quadratic model for yield (Fig.3B), FPF (Fig.3D), and nanoparticle size (Fig.3E), and 2FI model for MMAD (Fig.3C). The optimized process parameters (CPPs) were 100ºC inlet temperature, 100% aspirator, and 6mL/min feed rate (Fig.3F) resulting in an outlet temperature of 36ºC (< Tg of PLGA). The dry powder contained 20µg of BDQ and 80µg of PTD (per mg) and moisture content < 1%w/w for all BBD runs and were excluded from DoE analysis. Further, the optimized dry powder had a yield of 72±2%, excellent aerosolization performance (MMAD 2.13µm and FPF >80%; an average of BDQ and PTD drug deposition in different NGI stages), and nanoparticle size of 338±24 nm (Fig.3F).
Conclusion: The BDQ-PTD combination showed an additive effect for M.tb inhibition and was successfully loaded into polymeric nanoparticles. In addition, the QbD approach helped optimize process parameters and develop dry powder with a suitable quality profile for inhalation delivery in TB patients.
Acknowledgements: This study was supported by funds provided to NKK by the Department of Pharmaceutical Sciences and College of Pharmacy and Health Sciences (CPHS), St. John’s University.
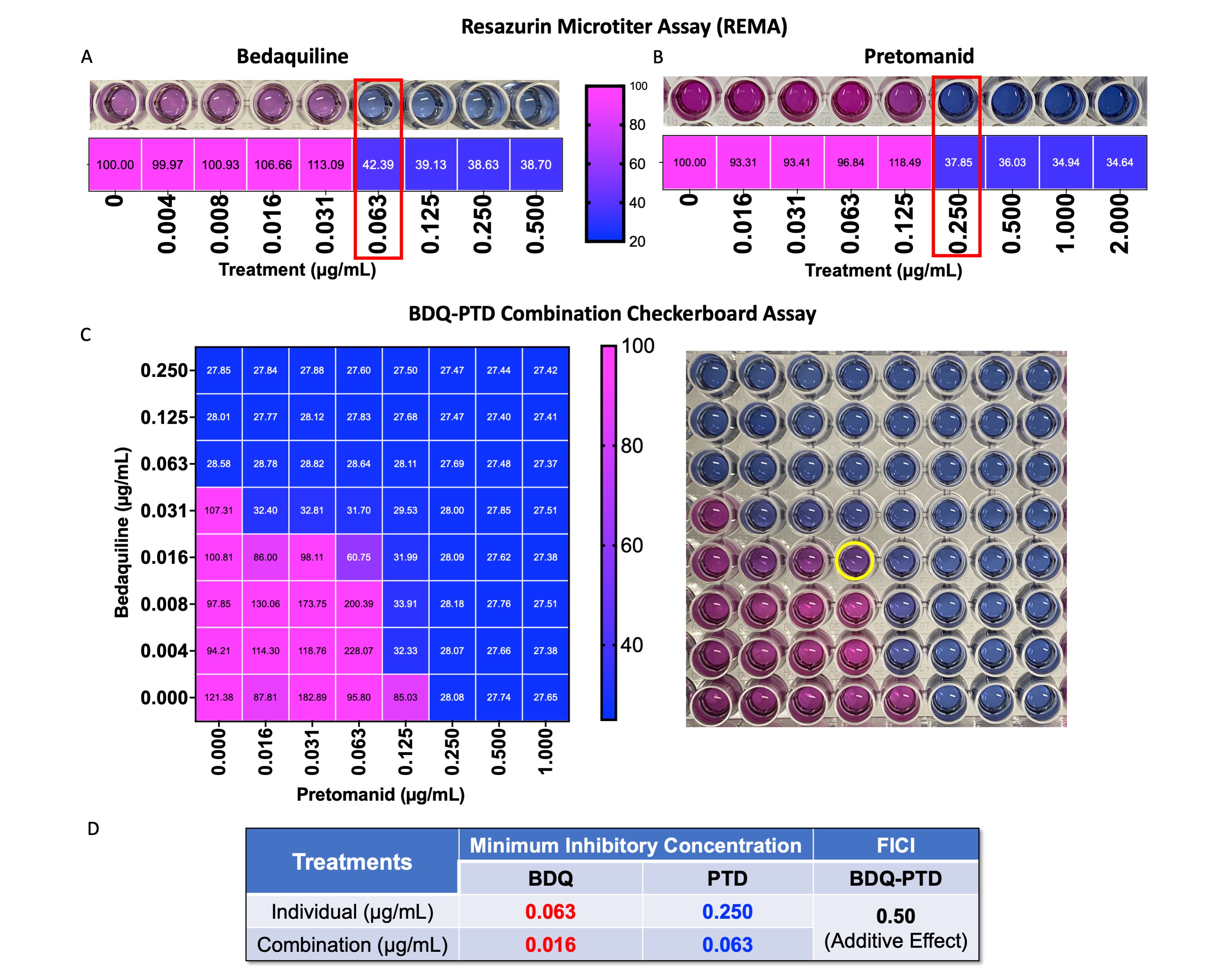 Minimum inhibitory concentration (MIC) of (A) Bedaquiline (BDQ) and (B) Pretomanid (PTD) were estimated using REMA (lowest concentration at which color in well changes from pink to blue and bacterial viability < 70%). (C) MIC estimation of BDQ-PTD combination using checkerboard assay and visualized by bacterial viability heatmap. (D) MIC values for individual drugs and combination and FIC index showing additive effect.
Minimum inhibitory concentration (MIC) of (A) Bedaquiline (BDQ) and (B) Pretomanid (PTD) were estimated using REMA (lowest concentration at which color in well changes from pink to blue and bacterial viability < 70%). (C) MIC estimation of BDQ-PTD combination using checkerboard assay and visualized by bacterial viability heatmap. (D) MIC values for individual drugs and combination and FIC index showing additive effect. 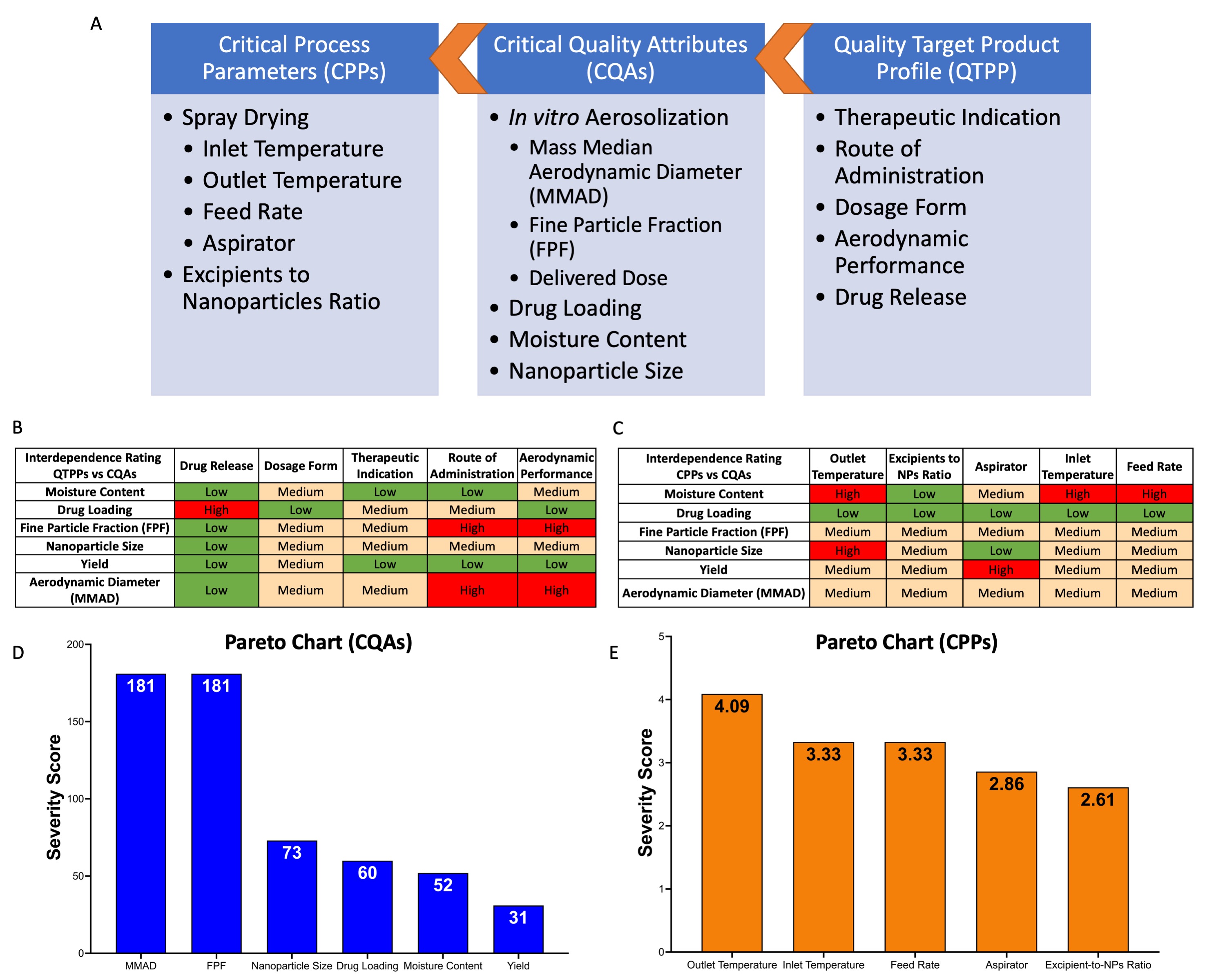 (A) Quality-by-design roadmap for developing dry powder inhaler products by defining QTPPs, CQAs, and CPPs. Interdependence rating for (B) the QTPPs and CQAs and (C) the CPPs and CQAs. Pareto charts of the (D) CQAs and (E) CPPs with severity scores computed by the software.
(A) Quality-by-design roadmap for developing dry powder inhaler products by defining QTPPs, CQAs, and CPPs. Interdependence rating for (B) the QTPPs and CQAs and (C) the CPPs and CQAs. Pareto charts of the (D) CQAs and (E) CPPs with severity scores computed by the software.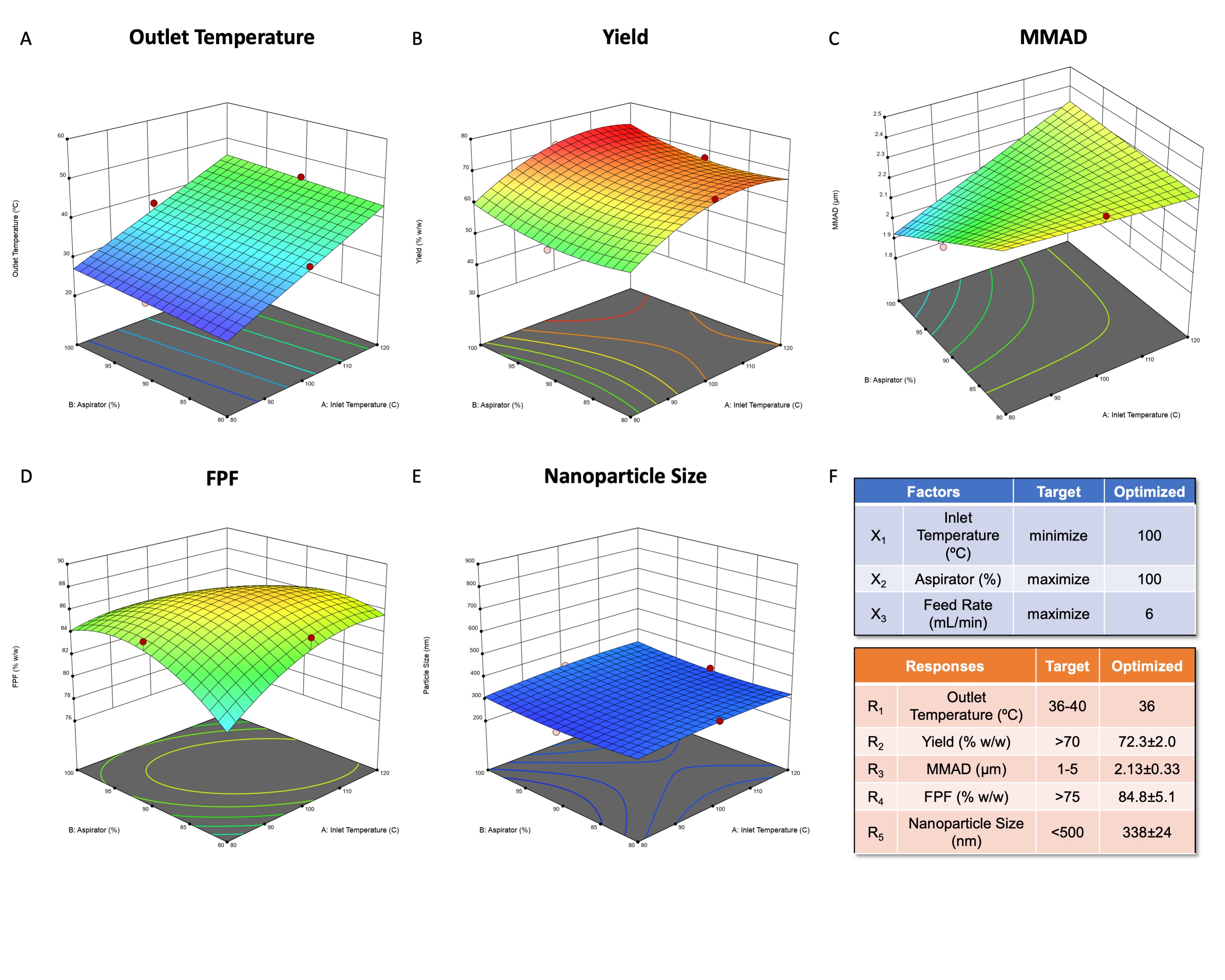 Three-dimensional (3D) response surface plots of (A) outlet temperature, (B) yield, (C) MMAD, (D) FPF, and (E) nanoparticle size as a function of X1: inlet temperature and X2: aspirator with X3: feed rate set at 6 mL/min. (F) Optimized process parameters and quality attributes (responses R1-5) for dry powder prepared using optimized parameters with defined target values resulting in a desirability value of 0.85.
Three-dimensional (3D) response surface plots of (A) outlet temperature, (B) yield, (C) MMAD, (D) FPF, and (E) nanoparticle size as a function of X1: inlet temperature and X2: aspirator with X3: feed rate set at 6 mL/min. (F) Optimized process parameters and quality attributes (responses R1-5) for dry powder prepared using optimized parameters with defined target values resulting in a desirability value of 0.85.