Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M0930-08-50) Design, Development, and Evaluation of Novel Extended-Release Liposomal Topical Cream Formulation of Pregabalin Using Microemulsion Technique for the Treatment of Local Neuropathic Pain
Monday, October 23, 2023
9:30 AM - 10:30 AM ET

Chetan Chure, M.Pharm. (he/him/his)
Senior Manager
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada
Chetan Chure, M.Pharm. (he/him/his)
Senior Manager
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada- VK
Varun Kumar Koleti, M.Pharm. (he/him/his)
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada - MT
Mallika Tushakiran, M.Pharm. (she/her/hers)
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada - LZ
Liang Zhang, Ph.D. (he/him/his)
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada - BL
Brian Lankadurai, Ph.D. (he/him/his)
Contract Pharmaceuticals Ltd.
Mississauga, Ontario, Canada
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Neuropathic pain (NP) is defined as a painful condition caused by neurological lesions or diseases. NP remains both prevalent and challenging to treat [1]. It often occurs in patients with neurological disorders, such as Multiple sclerosis, diabetic peripheral neuropathy, postherpetic neuralgia (“long COVID” symptoms), spinal cord injury, stroke, or other conditions. For NP, Pregabalin (PG) is the most prescribed drug by physicians. PG is currently available in oral dosage forms like tablets, capsules, and oral solutions [2]. For the convenience of the patients who cannot take oral medication of PG, a topical extended-release cream formulation of PG with an advanced liposome approach using a prominent nano-emulsion technique was prepared and its physical and chemical efficiency was evaluated. The PG was loaded in the liposomal bilayers (Oil phase) acting as an extended-release source and PG is also dissolved in the continuous phase acting as an immediate release mechanism so that it can penetrate effectively into the deeper skin layers. The target is to experience greater relief for the NP with this novel liposomal cream
Methods: PG liposomal cream was prepared by using different emulsion techniques. The raw materials were selected to prepare the liposome bilayers, to increase the solubility of the PG in the liposomal bilayers as well as penetration into the skin. Hence, the development work started with the solubility trials of the PG in different solvent systems such as Isopropyl myristate, Propylene glycol, Glycerin, Diethylene glycol, and water. L-α-phosphatidylcholine (95%) (Soy) was used as a phospholipid and cholesterol as stabilizing agent for the lipids. Cream base forming agents like Cetyl alcohol and Cetostearyl alcohol were used. The different emulsifiers like, Myrj S40 SPAN80, and Tween 80 were selected for the water in oil (W/O), oil in water (O/W), and water in oil in water (W/O/W) emulsions respectively. Benzyl alcohol was used as a preservative (Table-1 List of materials and function). In all three batches, the oil phase and aqueous phase ingredients were heated to 70-80°C separately, PG was added to the oil and aqueous phase and mixed until dissolved. An emulsifier was added to the oil phase to create W/OW or O/W emulsion. Both phases were emulsified for the required amount of time and then cooled down under mixing to room temperature (20-25°C). The first batch (F0) and the third batch (F2) were prepared with W/O/W and O/W emulsion techniques respectively, using Silverson® L5M-A homogenizer at 10000 RPM. The second batch (F1) was prepared using the W/O/W emulsion technique using Microfluidics™ M110P high pressure Microfluidizer® at 25000 psi pressure under high temperature (50-60°C).
Results: We selected and tested the formulation for Critical Quality Attributes like description, pH, liposome morphology, PG assay, and phase separation under stress conditions (50°C for 5 days). The description was uniform, smooth, and a homogenous white color cream. pH was between 6.30 to 6.60. The initial assay results of the active were consistent throughout the formulations developed. The formulation was found to be stable for the period studied, no phase separation of the emulsion was observed until one week at 50°C (Table-2: Analytical results of the optimized formulation, Figure-1: A-D for phase separation study photographic images). The macromolecular structure of the liposomes was observed using Nikon Eclipse microscope using 10x, 40x, and 100x objective lenses (Figure-1: E-M). The morphology of the prepared liposomes was characterized using SEM - Quattro S ESEM by Thermoscientific equipment (Figure-2: N-V). The size range observed in the SEM was between 5-30µm. For SEM, Peltier Cold Stage, using a wet method at 2-5°C, Pressures between 200-400Pa were used to image. Round spherical-shaped liposomes are clearly visible under SEM.
Conclusion: PG extended-release topical liposomal cream formulation has been successfully formulated and optimized with physicochemical characteristics and maintained stability for 5 days under stress conditions. This study entails a promising alternative to the oral formulations of PG for reducing NP treatment in adults. The proposed formula is the potential to be tested in IVRT and IVPT studies owing to the improved safety profile as compared to the oral solid dosage forms of PG.
References: [1] Mitsikostas D, Moka E, Orrillo E, et al. (February 20, 2022) Neuropathic Pain in Neurologic Disorders: A Narrative Review. Cureus 14(2): e22419. doi:10.7759/cureus.22419
[2] https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
[3] Wang, J., Zhang, L., Chi, H., & Wang, S. (2016). An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid-polymer hybrid nanoparticles for local anesthetic therapy. Drug delivery, 23(4), 1254–1260.
[4] Formulation of water-in-oil-in-water (W/O/W) emulsions containing trans-resveratrol
J. Wang, A. Shi, D. Agyei and Q. Wang, RSC Adv., 2017, 7, 35917 DOI: 10.1039/C7RA05945K
Acknowledgements: We want to thank McMaster University, Canada for providing access to the ESEM in support of this project.
.jpg) Table 1: List of raw materials used for the study and function
Table 1: List of raw materials used for the study and functionTable 2: Analytical results of the optimized formulation
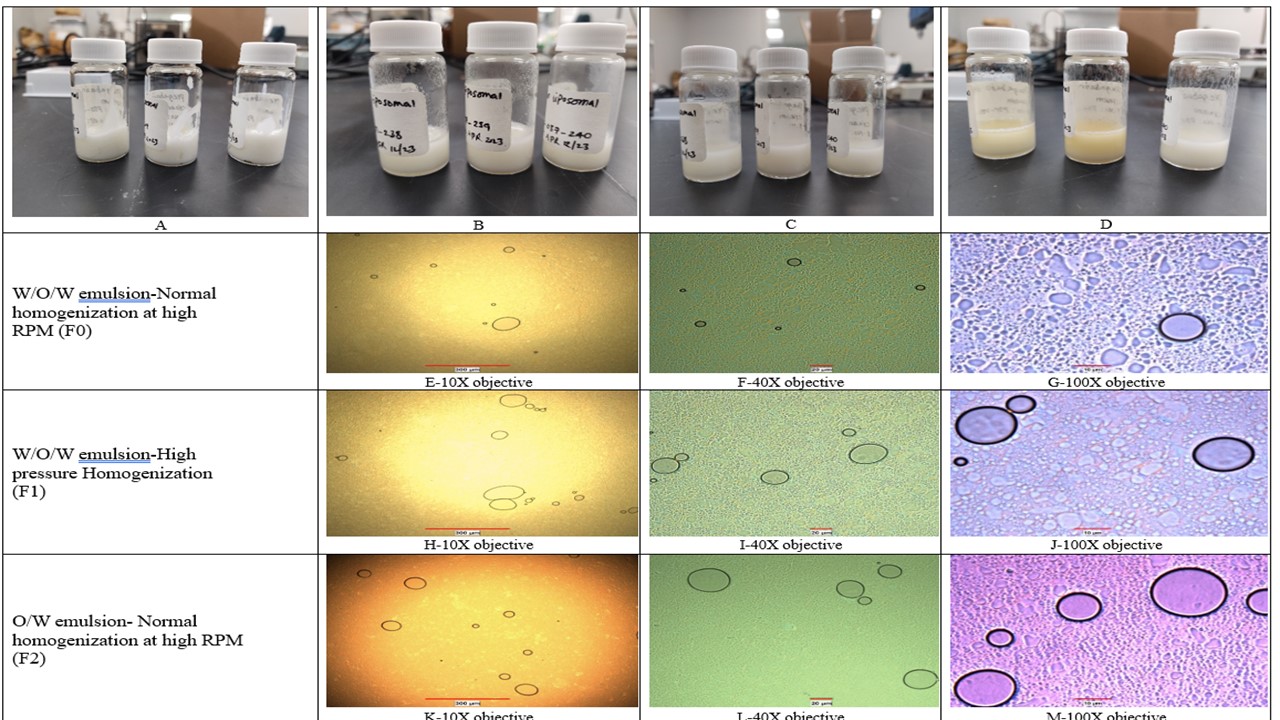 Figure-1: Photographic images of phase separation stress study at 50°C the PG liposomal cream formulation A: Initial-T0 B: First day at 50°C-No separation observed-T1 C: Second day at 50°C-No separation observed-T2 D: Fifth day at 50°C-No separation observed, however slight change in color from white to slight yellow observed-T5 E-G: Microscopy image of F0 formulation H-J: Microscopy image of F1 formulation K-M: Microscopy image of F2 formulation
Figure-1: Photographic images of phase separation stress study at 50°C the PG liposomal cream formulation A: Initial-T0 B: First day at 50°C-No separation observed-T1 C: Second day at 50°C-No separation observed-T2 D: Fifth day at 50°C-No separation observed, however slight change in color from white to slight yellow observed-T5 E-G: Microscopy image of F0 formulation H-J: Microscopy image of F1 formulation K-M: Microscopy image of F2 formulation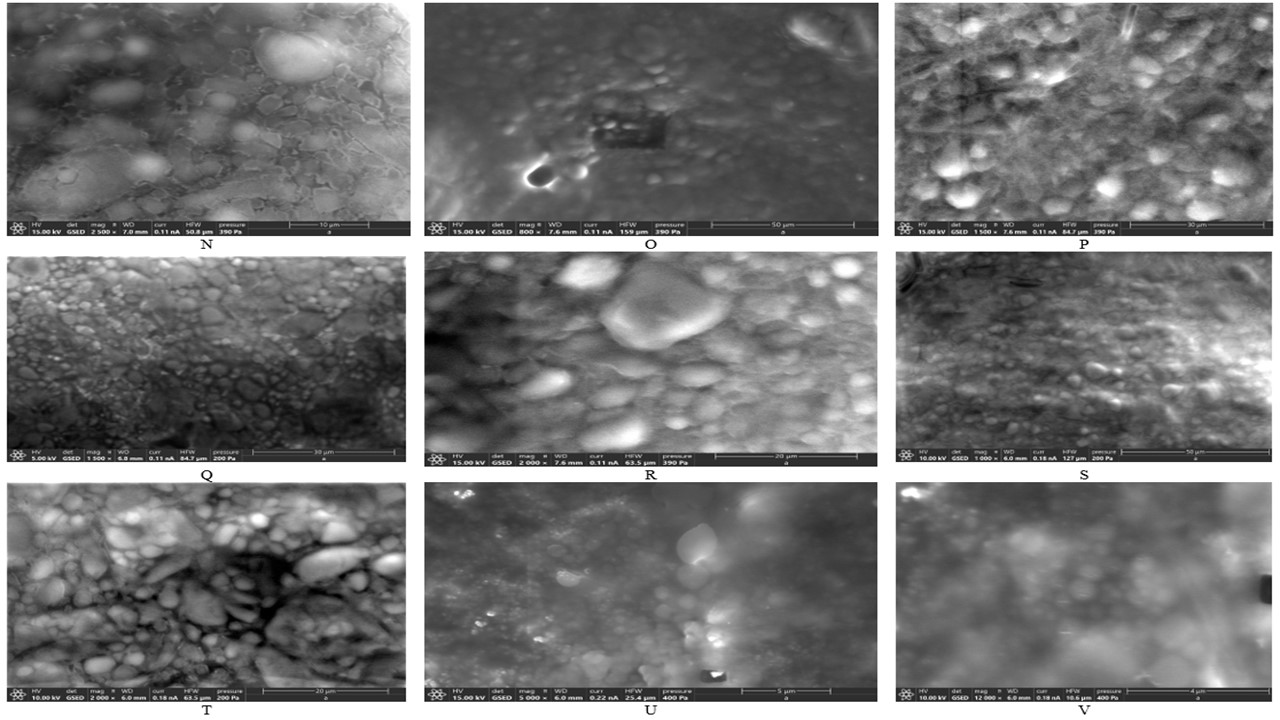 Figure-2: SEM images of PG liposomal cream formulation using Quattro S ESEM (Make: Thermoscientific) equipment, Peltier Cold Stage, Wet method at 2-5°C, Pressures between 200-400Pa. N-P: SEM images of F0 formulation-prepared with W/O/W emulsion technique at high RPM homogenization. Q-S: SEM images of F1 formulation-prepared with W/O/W emulsion technique at high pressure homogenization. T-V: SEM images of F2 formulation-prepared with O/W emulsion technique at high RPM homogenization.
Figure-2: SEM images of PG liposomal cream formulation using Quattro S ESEM (Make: Thermoscientific) equipment, Peltier Cold Stage, Wet method at 2-5°C, Pressures between 200-400Pa. N-P: SEM images of F0 formulation-prepared with W/O/W emulsion technique at high RPM homogenization. Q-S: SEM images of F1 formulation-prepared with W/O/W emulsion technique at high pressure homogenization. T-V: SEM images of F2 formulation-prepared with O/W emulsion technique at high RPM homogenization.