Formulation and Delivery - Chemical
Category: Poster Abstract
(W1130-02-09) Development of Polymeric Nanoparticles for Targeted Follicular Delivery of Tazarotene for Treatment of Inflammatory Skin Conditions
Wednesday, October 25, 2023
11:30 AM - 12:30 PM ET

Sharvari Kshirsagar, M.Pharm. (she/her/hers)
Graduate Student
Mercer University
Atlanta, Georgia, United States
Sharvari Kshirsagar, M.Pharm. (she/her/hers)
Graduate Student
Mercer University
Atlanta, Georgia, United States- NS
Nisha Shrestha, B.Pharm. (she/her/hers)
Mercer University
Atlanta, Georgia, United States 
Thomas Kipping, Ph.D. (he/him/his)
Head of Drug Carriers
MilliporeSigma a Business of Merck KGaA
Darmstadt, Hessen, Germany
Ajay Banga, Ph.D.
Professor and Chair
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Acne vulgaris and plaque psoriasis are the most common inflammatory skin conditions with an estimated 117.4 million cases of acne vulgaris worldwide and 64.6 million cases of psoriasis (1,2). Acne vulgaris is a condition of the pilosebaceous units or hair follicles characterized by inflamed nodules, pustules, and papules with scarring. Plaque psoriasis is a chronic immune-mediated disease caused by the hyperproliferation of keratinocytes with infiltration of T-lymphocytes and release of cytokines. Topical retinoids are prescribed as the first-line treatment for moderate-to-severe acne vulgaris and plaque psoriasis. Tazarotene is a third-generation retinoid prodrug which, converts to tazarotenic acid, binds to retinoic acid receptors (RAR) β and γ, thereby modulating keratinocytes differentiation, reduction in keratinocyte proliferation and decreased expression of inflammatory markers. However, tazarotene causes skin irritation, dryness, flaking, and photosensitivity. It is crucial to target follicular delivery of tazarotene for higher contact and bioavailability at the site of action. The goal of the present study is to incorporate tazarotene into polymeric nanoparticles to increase its follicular delivery with a prolonged residence time, thereby eliminating the peripheral effects on the skin and increasing the success of the treatment.
Methods: The overall schematic of the objective and methodology is presented in Figure 1. A 90% saturated solution of tazarotene in propylene glycol (PG) was used as a donor solution for the passive in vitro permeation (IVPT) with maximum thermodynamic activity, applied with finite dose on full-thickness porcine skin (n=4) by rubbing using an inverted vial for 30 seconds. Tazarotene amount in hair follicles was determined by differential tape stripping technique. After 24 h, the unabsorbed formulation was removed, 15 D-Squame tapes were applied to remove the stratum corneum. Next, a drop of cyanoacrylate glue was applied on the skin with a D-Squame tape with light pressure for 15 seconds, followed by 5 min for polymerization of glue. The tape was immediately removed to uproot the hair follicles. Tazarotene was extracted from the skin, cyanoacrylate glue and the tape strips and analyzed using a validated Ultra Performance Liquid Chromatography (UPLC) method. We further encapsulated tazarotene in different grades of PLGA (EXPANSORB® DLG 50-5A and 50-8A) to form nanoparticles via nanoprecipitation technique. Tazarotene and PLGA were dissolved in acetone and injected into the aqueous phase (1% w/w polyvinyl alcohol EMPROVE®18-88) at 1 mL/min rate and stirred at 800 RPM, followed by overnight stirring for acetone evaporation. The nanoparticles were collected by centrifugation, washed, and lyophilized with cryoprotectant trehalose to obtain dried nanoparticles. Preliminary trials were conducted to determine the PVA concentration, phase ratio, and solvent evaporation time. Four batches of nanoparticles with different drug: polymer ratios were prepared and characterized for particle size, percent drug loading, encapsulation efficiency and scanning electron microscopy (Figure 2). F3 was selected for IVPT to determine skin and follicular delivery of tazarotene.
Results: The passive permeation results showed no receptor delivery, attributed to the highly lipophilic nature of tazarotene. The follicular delivery was found to be 0.41 ± 0.06 µg/cm2 as determined by differential tape stripping technique and the total skin delivery was found to be 3.17 ± 0.16 µg/ cm2 (Figure 3A). The four optimized batches of nanoparticle formulations were prepared and analyzed where the percent drug loading increased with decreasing polymer ratio. Batch F3 showed the lowest and most uniform particle size, as observed by the Dynamic Light Scattering (Zetasizer), along with a good encapsulation efficiency (EE). The nanoparticles were easily redispersible in PG due to lyophilization with good content uniformity, as analyzed on UPLC. SEM results indicated that the nanoparticles were spherical in shape and uniform in size. IVPT results of the nanoparticle group showed a significant increase (p= 0.0194) in both follicular delivery (0.97 ± 0.23 µg/cm2) as well as significant increase (p=0.0166) in total skin delivery (6.27 ± 0.76 µg/cm2) as compared to passive delivery (Figure 3B). Rubbing the nanoparticles during application resulted in mobilization of the hair follicle, assisting in the follicular uptake. Nanoparticles resulted in higher accumulation in the hair follicles by aggregation in the follicular opening and the duct, in addition to the stratum corneum via close contact with superficial junctions and furrows. Thus, tazarotene nanoparticles increased the follicular delivery two folds by entering via a shunt pathway, thereby increasing the total delivery into the skin.
Conclusion: Tazarotene-loaded nanoparticles were prepared with PLGA using nanoprecipitation technique and characterized successfully. The optimized nanoparticles had the desired particle size, distribution, appearance, and drug loading. Nanoparticles showed a significantly higher follicular delivery and overall higher skin absorption of tazarotene as compared to passive delivery. Thus, the feasibility of developing a topical nanoparticle-based formulation of tazarotene to achieve higher follicular and skin delivery with potentially reduced side effects was demonstrated with this study.
References: 1. H. Chen, T.C. Zhang, X.L. Yin, J.Y. Man, X.R. Yang, M. Lu, Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: an analysis from the Global Burden of Disease Study 2019, British Journal of Dermatology, Volume 186, Issue 4, 1 April 2022, Pages 673–683
2. AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020 May;59(5):566-571. doi: 10.1111/ijd.14864. Epub 2020 Apr 6. PMID: 32250451.
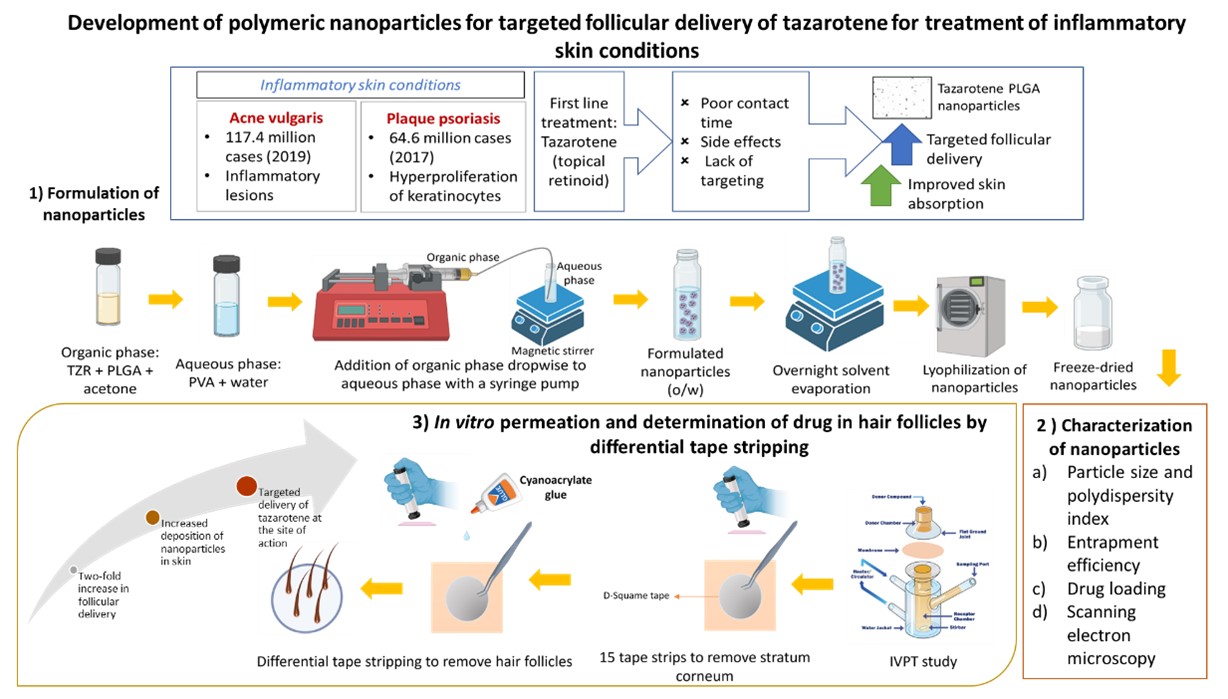 Schematic representing the overall objective, methodology of nanoparticle formation, in vitro permeation, differential tape stripping, and study results in summary.
Schematic representing the overall objective, methodology of nanoparticle formation, in vitro permeation, differential tape stripping, and study results in summary.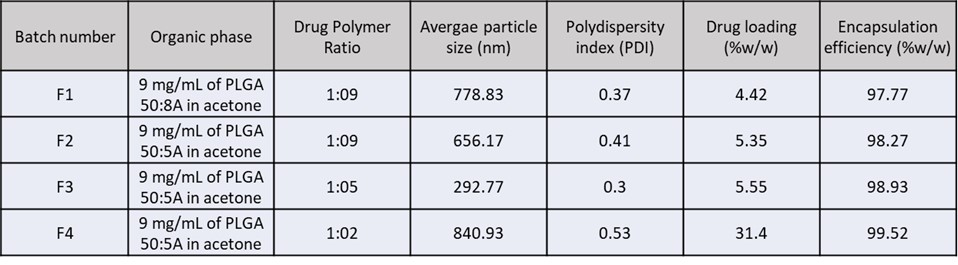 Summary of the optimized formulations prepared and analyzed for tazarotene nanoparticles. Particle size and PDI was measured (n=3) using dynamic light scattering Zetasizer. Drug loading and encapsulation efficiency (n=3) analyzed using validated UPLC method
Summary of the optimized formulations prepared and analyzed for tazarotene nanoparticles. Particle size and PDI was measured (n=3) using dynamic light scattering Zetasizer. Drug loading and encapsulation efficiency (n=3) analyzed using validated UPLC method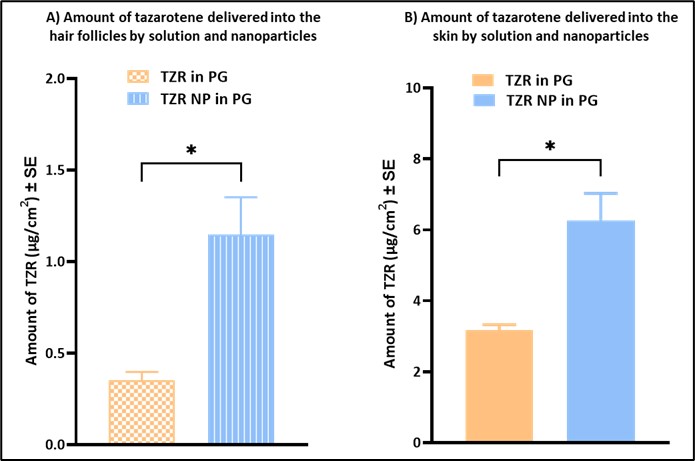 Results of the in vitro permeation study showing: A) follicular delivery of tazarotene from solution control and nanoparticle group and (B) total skin delivery of tazarotene by solution control and nanoparticle group using full-thickness porcine skin. Statistical analysis performed using unpaired t test. * indicates significant difference (p < 0.05)
Results of the in vitro permeation study showing: A) follicular delivery of tazarotene from solution control and nanoparticle group and (B) total skin delivery of tazarotene by solution control and nanoparticle group using full-thickness porcine skin. Statistical analysis performed using unpaired t test. * indicates significant difference (p < 0.05)