Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(T1530-08-55) Investigation Study of Color Change in Drug Tablets by Raman Spectroscopy
Tuesday, October 24, 2023
3:30 PM - 4:30 PM ET

Zhouming Zhao, PhD
Executive Vice President, Pharmaceutical Research Institute
Zhejiang Huahai Pharmaceutical Co., Ltd.
Mountain Lakes, New Jersey, United States- DL
Donghao Liu, Ph.D.
Zhejiang Huahai Pharmaceutical Co, Ltd.
Linhai, Zhejiang, China (People's Republic) - LZ
Lijun Zhang, MS
Zhejiang Huahai Pharmaceutical Co, Ltd.
Linhai, Zhejiang, China (People's Republic) - JL
Jinping Lu, MS
Zhejiang Huahai Pharmaceutical Co, Ltd.
Linhai, Zhejiang, China (People's Republic) - YL
Yuxin Li, MS
Zhejiang Huahai Pharmaceutical Co, Ltd.
Linhai, Zhejiang, China (People's Republic) - WH
Weiling He, Ph.D.
Zhejiang Huahai Pharmaceutical Co, Ltd.
Linhai, Zhejiang, China (People's Republic)
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: In-depth knowledge of impurities can help to control the impurities of the drug product more effectively in the formulation development. Recently, a case of color change occurred in the stress study of the complex double-layer tablets from light yellow to pink or red brown. And one of the most puzzling aspects was that the color variated in different regions on the tablet. Therefore, a series of experiments were performed to investigate the types of impurities and the root cause in order to improve the formulation process.
Methods: (1) In the double-layer tablet, the active pharmaceutical ingredient (API) exists in layer A (API layer) with some excipients and the other majority of excipients are in the layer B (the 2nd layer).
(2) Stress study: (a) the tablets were placed into the sealed container under 60 ℃/75% RH for 5 days. (b) According to the true proportion in the tablets, the mixed powders of API and excipients were placed into the sealed container under 60 ℃/75% RH for 5 days. (c) Each tablet was sealed in a blister individually under 60 ℃/75% RH for 5 days.
(3) Microscope-Raman spectrometer manufactured by Renishaw, laser wavelength: 785 nm, laser power: 10%, detector: charge coupled device, map scanning area: red square region in Fig.1.
Results: As part of color investigation, the suspected impurities were initially expected to be identified through high performance liquid chromatography (HPLC) - mass spectrometry (MS). Only the largest impurity peak of impurities (Imp A) was identified, and no new unidentified impurity peak appeared in the chromatogram. Thus, the impurities were further examined by microscope - Raman spectrometer because of its high resolution, in situ analysis, nondestructive analysis(1, 2). The double - layer tablet was cut into sections from two directions (vertically and horizontally) for better comparison with the tablet surface (Fig. 1A-a). In Fig.1A-b, the color of API layer (on the surface touching with the 2nd layer) was the darkest compared with that of the whole tablet surface of API layer, and the color of the vertical section of API layer was barely changed in Fig.1A-c. A special spectrum (Fig. 1B) at Ramen shift of 815 cm-1, which did not belong to API, excipients, and any known impurities, was only found on the surface of API layer. The results of stress study among API and excipients were shown in Fig.2 by HPLC and Raman spectra. Imp A increased significantly in test VI and VII, and pink color impurities appeared in test VI. The excipients A and B contained Mg and Al, respectively. The peak at 815 cm-1 were also found in these two tests. Therefore, the impurities were indicated to be the metal coordinated complex with API or Imp A. Fig.1C showed that the spectrum of standard substance of coordinated complex of Mg and Imp A (Mg-Imp A) was similar to that of test VI and VII. The characteristic peak of 815 cm-1 is related to metal - oxygen bond because the Raman spectra cannot discriminate different metals. Moreover, the reasonable mechanism showed in Fig.3 clarified the reason. The coordinated complex impurities could be formed on the contact surface while Imp A in API layer touch metal element in excipients of the 2nd layer. Based on the investigation finding, it would be careful to minimize the chances between layer A and layer B of tablets when choosing product package. With that in mind, the individual tablet package was selected to decrease the exposure risk among tablets. The results showed that the spectra of coordinated complex on the surface of the whole tablet were not found with individual packaging. Accordingly, the packaging was a critical quality attribute (CQA) in the production process.
Conclusion: The coordinated complex impurities were formed in certain situation or condition and could be overlooked in quality analysis. In this study, the metal coordinated complex impurities were found in double-layer tablets by microscope - Raman spectrometer. A reasonable formation route was inferred. According to Quality by Design (QbD), the finished product should be packaged individually in blister instead of bulk container.
References: 1. Xu G, Song P, Xia L. Examples in the detection of heavy metal ions based on surface-enhanced Raman scattering spectroscopy. Nanophotonics. 2021;10(18):4419-45. doi: 10.1515/nanoph-2021-0363.
2. Hu W, Xia L, Hu Y, Li G. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchemical Journal. 2022;172. doi: 10.1016/j.microc.2021.106908.
Acknowledgements:The authors declare no conflict of interest.
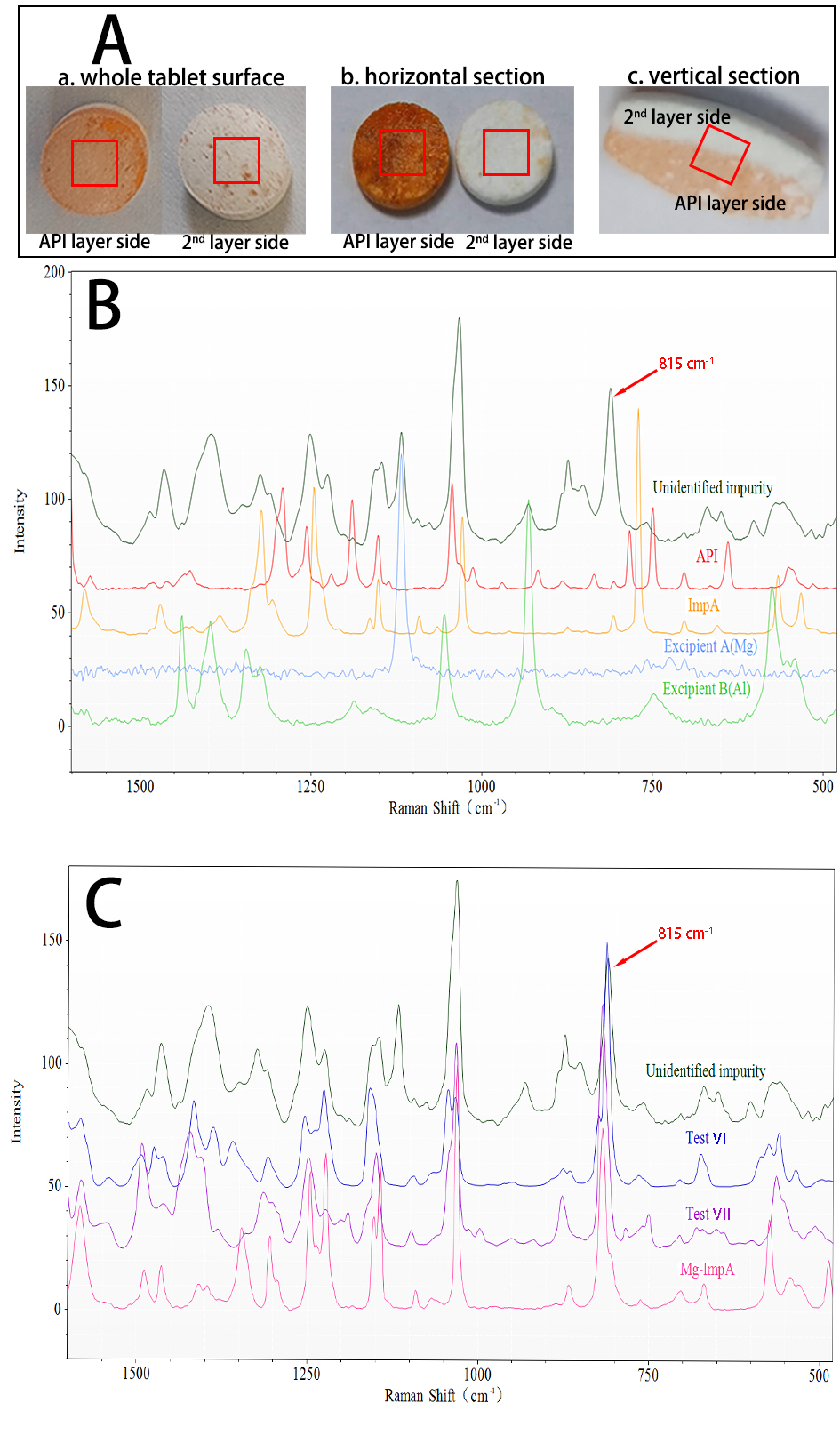 Fig.1 (A) The comparison of three conditions of double-layer tablets (a, both sides of the whole double-layer tablet, b, horizontal section (separated layers) of the tablet, c, vertical section of the tablet); (B) The Raman spectra of API, Imp A, excipients and unidentified impurity; (C) The Raman spectra of Mg-Imp A, Test VI, Test VII and unidentified impurity
Fig.1 (A) The comparison of three conditions of double-layer tablets (a, both sides of the whole double-layer tablet, b, horizontal section (separated layers) of the tablet, c, vertical section of the tablet); (B) The Raman spectra of API, Imp A, excipients and unidentified impurity; (C) The Raman spectra of Mg-Imp A, Test VI, Test VII and unidentified impurity.jpg) Fig.2 The results of stress study
Fig.2 The results of stress study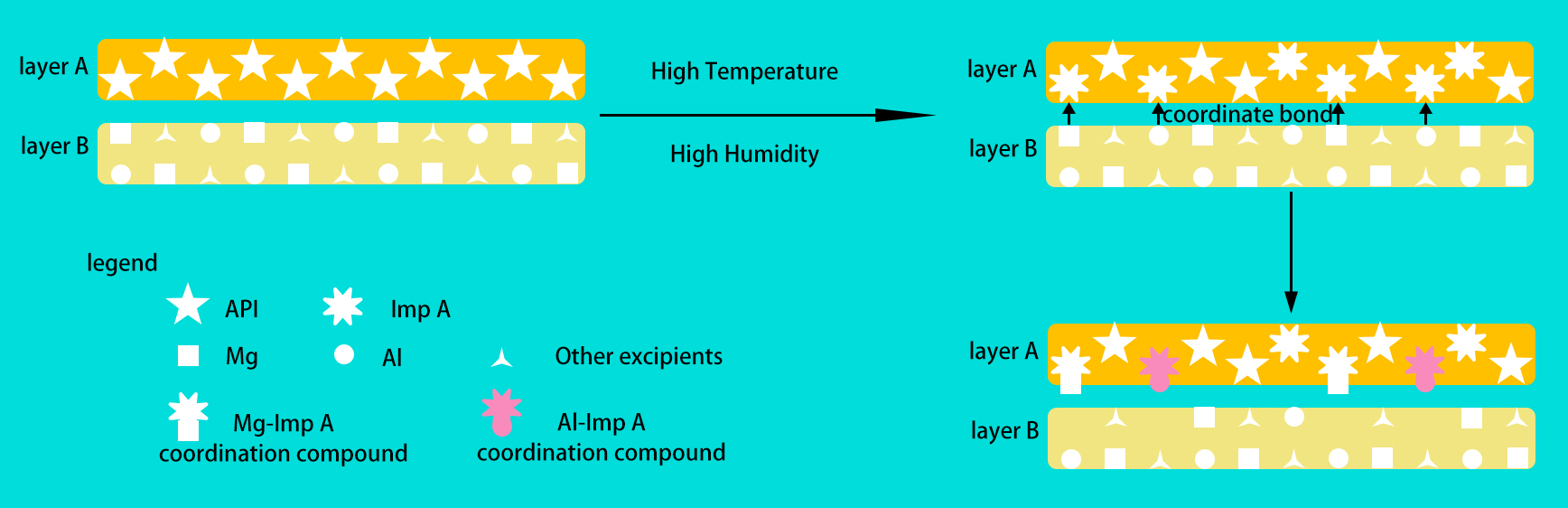 Fig.3 The formation mechanism of impurities
Fig.3 The formation mechanism of impurities