Formulation and Delivery - Chemical
Category: Poster Abstract
(T0930-11-75) Role of PLGA Variability in Controlled Drug Release from Dexamethasone Intravitreal Implants
Tuesday, October 24, 2023
9:30 AM - 10:30 AM ET
- FZ
Feng Zhang, Ph.D. (he/him/his)
University of Texas at Austin
Austin, Texas, United States 
Mark Costello, BS
PhD Student
University of Texas at Austin
Austin, Texas, United States- JL
Joseph Liu
University of Texas at Austin
Austin, Texas, United States - LK
Louise Kuehster, BS
University of Texas at Austin
Austin, Texas, United States - YW
Yan Wang, Ph.D. (she/her/hers)
Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States - BQ
Bin Qin, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - NL
Nathaniel Lynd, Ph.D.
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Poly(lactic-co-glycolic acid) (PLGA) is a biodegradable copolymer commonly applied to control drug release in long-acting injectable formulations. To date, more than 20 PLGA–based drug products have been approved by the FDA, with the first approval dating back to 1989. (1) Interestingly, there are no generic PLGA–based formulations available in the USA despite several of these products extending beyond their exclusivity period, largely due to challenges matching the release profile of the reference listed drug (RLD). (2) One such product near the end of its exclusivity period is Ozurdex (dexamethasone intravitreal implant) indicated for treatment of macular edema and noninfectious uveitis. (3) In this study, dexamethasone intravitreal implants equivalent to Ozurdex were manufactured using PLGAs from different sources to evaluate the impact of polymer variability on controlled drug release of dexamethasone in vitro.
Methods: Dexamethasone intravitreal implants with structural characteristics like Ozurdex were prepared with the following composition: 60% micronized dexamethasone, 30% 50:50 acid-terminated PLGA, and 10% Resomer RG 502 (50:50 ester-terminated PLGA). Four unique lots of 50:50 acid-terminated PLGA were used to prepare four unique implants for experimentation. Two of the lots were sourced from Evonik: commercially available Resomer RG 502H. The remaining two lots were sourced from Akina: a custom-synthesis of two PLGAs with different monomer ordering (blockiness). In vitro release testing was performed on the four implant formulations in both normal saline and phosphate-buffered saline (PBS, pH 7.4) at 37°C to evaluate drug release over several weeks.
Results: Characterization of the four unique lots of acid-terminated PLGA used to prepare the implants showed they exhibited similar molecular weight distribution, glass transition temperature, and moisture content prior to implant preparation (Figure 1). Polymer source had no impact to implant structural properties during manufacture using a hot-melt extrusion and shaping process (Figure 2). In vitro release testing in normal saline showed no differences in the release profile of the four formulations. Release testing in PBS showed an extended duration of release and significantly different release profiles (Figure 3).
Conclusion: Dexamethasone intravitreal implants similar to Ozurdex were produced using four unique lots of acid-terminated PLGA. In vitro release testing in normal saline showed no impact of PLGA variability to drug release. In vitro release testing in PBS significantly extended the duration of drug release and revealed major changes to the release profiles which may be attributed to residual acids left over from polymer synthesis and differences in PLGA blockiness. A rabbit eye study with these formulations will be performed to evaluate the impact of PLGA variability on drug release in vivo.
References: (1) Wang Y, Qin B, Xia G, Choi SH. FDA's Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021;23(4):92.
(2) Park K, Skidmore S, Hadar J, Garner J, Park H, Otte A, et al. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J Control Release. 2019;304:125-34.
(3) Highlights of Prescribing Information, OZURDEX (dexamethasone intravitreal implant) Madison, NJ: Allergan; 2020 [Available from: https://www.rxabbvie.com/pdf/ozurdex_pi.pdf.
Acknowledgements: This work was supported by the Broad Agency Announcement (BAA) Contract # 75F40120C00198 from the U.S. Food and Drug Administration (FDA). The content reflects the views of the authors and should not be construed to present FDA’s views or policies.
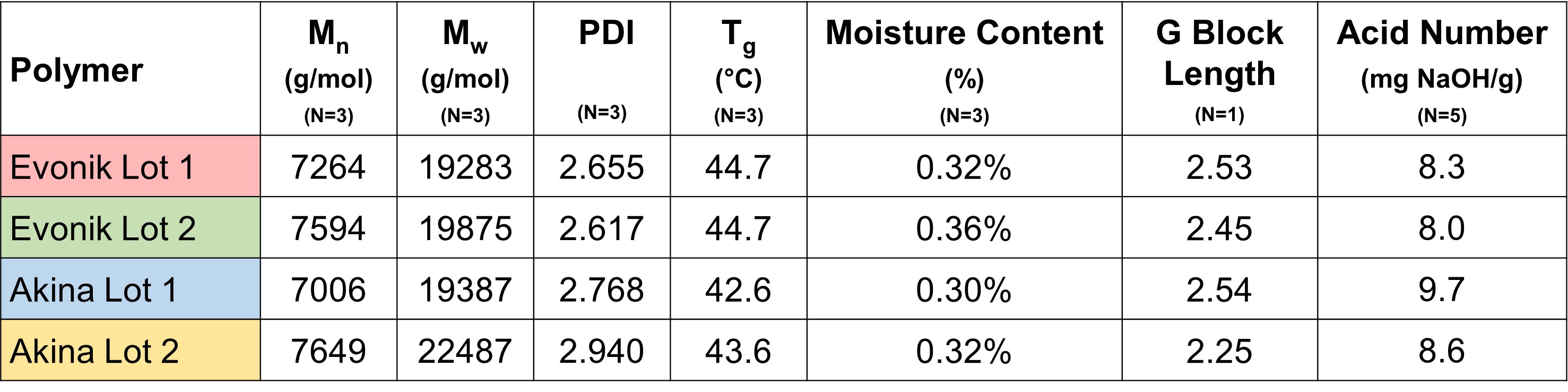 Figure 1. Properties of the four 50:50 acid-terminated PLGAs used in this study to prepare dexamethasone intravitreal implants.
Figure 1. Properties of the four 50:50 acid-terminated PLGAs used in this study to prepare dexamethasone intravitreal implants.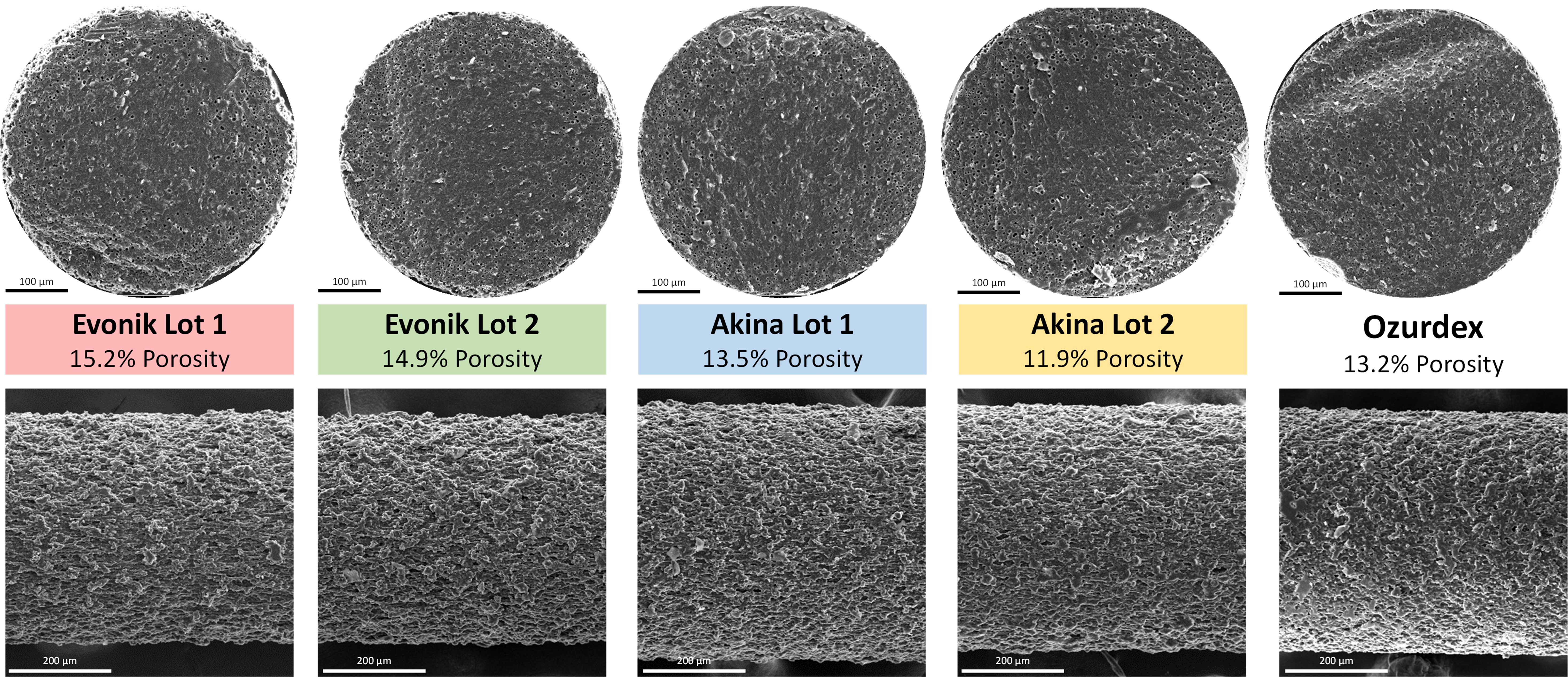 Figure 2. Scanning electron microscope images of Ozurdex and the four dexamethasone intravitreal implants produced in this study. Top row: cross-sections revealing internal porosity. Bottom row: profiles revealing surface morphology.
Figure 2. Scanning electron microscope images of Ozurdex and the four dexamethasone intravitreal implants produced in this study. Top row: cross-sections revealing internal porosity. Bottom row: profiles revealing surface morphology.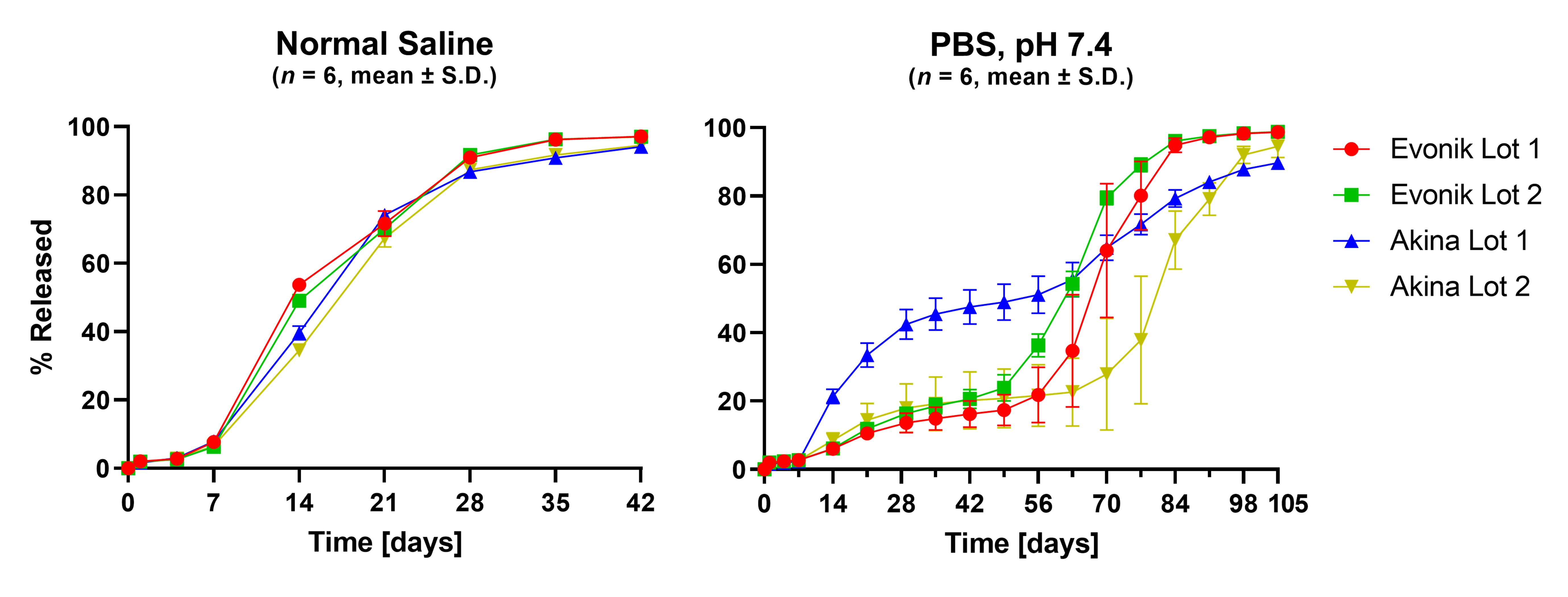 Figure 3. In vitro release profiles of the dexamethasone intravitreal implants in normal saline (left) and PBS, pH 7.4 (right).
Figure 3. In vitro release profiles of the dexamethasone intravitreal implants in normal saline (left) and PBS, pH 7.4 (right).