Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(M1330-08-53) Better Together: Thermally Unstable Drug Granulated Using a Thermal Process?
- AP
Adwait Pradhan, M.S. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - AP
Adwait Pradhan, M.S. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - BP
Brian Phillips
Ashland Specialty Ingredients
Wilmington, Delaware, United States - TB
Teslin Botoy, M.S. (she/her/hers)
Ashland Specialty Ingredients
Wilmington, Delaware, United States 
Fengyuan Yang, PhD (he/him/his)
Research Scientist
Ashland Specialty Ingredients
Wilmington, Delaware, United States- KK
Kapish Karan, M.S. (he/him/his)
Ashland Specialty Ingredients
Wilmington, Delaware, United States - TD
Thomas Dürig, Ph.D. (he/him/his)
Ashland Inc.
West Chester, Pennsylvania, United States - CM
Charlie Martin
Leistritz Extrusion
Branchburg, New Jersey, United States - FZ
Feng Zhang, Ph.D. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - BH
Brian Haight
Leistritz Extrisiom
Branchburg, New Jersey, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Twin-screw melt granulation (TSMG), a promising continuous manufacturing platform relies on heat and shear (dispersive and distributive mixing) at the kneading zone for granulation to occur. The kneading elements used in TSMG have a narrow tip clearance of 0.1 mm and a wider disc width of 5 mm to balance applied shear and elongational stresses during mixing and transport of materials. This high-shear, high-energy environment promotes drug degradation, amorphization, and particle size reduction1. Widening the tip clearance (0.2-0.4 mm) and using 2 mm narrow disc width elements may reduce peak shear and local heat generation, potentially leading to less drug degradation and a more stable final product. Preliminary work performed on mannitol-hydroxypropylcellulose melt granulation using a twin-screw extruder unleashed the potential of new kneading elements and elucidated how the lost peak shear with narrow disc and wider overflight clearance needs to be compensated by increasing staggering angle or degree of fill2. However, further research is needed to understand the complex interplay between the mixing efficiency, residence time distribution and degree of fill during the melt granulation process. This work builds upon that understanding to successfully formulate a stable granule of a thermally unstable gabapentin using a thermal and shear dominant process.
Methods: Gabapentin (GABA) is a high-dose and thermally unstable drug which requires granulation due to its poor compressibility. GABA and GABA-L content is quantified using HPLC with a sample concentration of 5 mg/mL and GABA-L limit of quantitation at 0.01%. As per USP, GABA-L has to be < 0.4% in final product. Klucel™ (Hydroxypropylcellulose) was chosen as the thermal binder due to its superior binding and tableting properties. Klucel level was fixed to 10% based on the degradation kinetics and preliminary granulation runs1. Granulation trials were carried out on a Leistritz ZSE-18 twin-screw co-rotating extruder with a 25 L/D barrel configuration having a 20 mm kneading block (10 elements staggered at 30°). Two different screw configurations were employed with 2 mm disc width elements and overflight clearance of 0.2, 0.4 mm respectively. Barrel temperature, screw speed and feed rate were used as the independent variables in a full factorial design for process optimization with % GABA-L and tensile strength as response variables. Tabletability of granules (250-600 µm) was evaluated using a STYL’one compaction simulator to compress granules at a compression pressure of 254 MPa. Granules were kept on stability at three conditions 25 °C/60% RH, 40 °C/75% RH, 40°C ambient humidity in induction sealed HDPE bottles without desiccant to assess GABA-L content during shelf-life.
Results: GABA degrades rapidly into GABA-L upon melting at 165 °C making thermal processing a challenging approach for granulation. A heat-cool-heat cycle run using a DSC confirmed the complete conversion of GABA to GABA-L upon melting. Granulation was conducted by varying barrel temperature from 90-120 °C, the screw speed from 30-200 rpm and feed rate from 2-3 kg/h. In alignment to mannitol melt granulation with the novel kneading elements, a higher barrel temperature of 120 °C was required for the onset of granulation and granule growth to offset the reduced viscous dissipation. Reduced peak shear with the new kneading elements was compensated for by increasing the degree of fill to promote granulation. This was achieved by running at lower screw speed of 30 rpm which also reduces heat generation at kneading zone. The value of running at lower screw speed has been established previously and confirmed with screw speed and barrel temperature having a significant impact on the % GABA-L during granulation (Table 1). Overflight clearance does not have a significant impact on drug degradation despite reducing peak shear (Table 1). % GABA-L content was observed to be below 0.04% (1/10th of the limit) for all the processing conditions employed in the design. The target tensile strength of 2 MPa was achieved only at high degree of fill and high barrel temperature of 120 °C (Figure 1). A linear extrapolation of the granule stability data at 40 °C, ambient humidity conditions for 6 months indicated a stable gabapentin product with a shelf life of 2 years equivalent to a commercial product (Figure 2). Despite low GABA-L content at lower barrel temperature, sufficient densification cannot be achieve highlighting the need for a detailed understanding of the complex interplay between binder level, local heat generation due to mixing and granule properties.
Conclusion: Despite being a thermally unstable and a high dose drug, a stable gabapentin product can be formulated using a melt granulation process with the new screw elements based on the granule storage stability data. The new kneading elements reduce the peak shear at kneading zone as observed from the high barrel temperature and high degree of fill required for granulation. However, the lost shear needs to be compensated with a high degree of fill as a minimum energy threshold needs to be met for granulation and tabletability improvement.
References: 1. Adwait Pradhan, Nada Kittikunakorn, Fengyuan Yang, Thomas Durig, Feng Zhang: Poster presentation on ‘How to maintain drug stability during twin-screw melt granulation?’ at AAPS 2022.
2. Adwait Pradhan, Brian Haight, Augie Machado, Charlie Martin, Mike Thompson, Feng Zhang: Poster presentation on ‘Effect of Kneading Disk Geometry on Twin-Screw Continuous Melt Granulation’ at AAPS 2022.
Acknowledgements: This project is funded by Ashland Specialty Ingredients, Wilmington, Delaware.
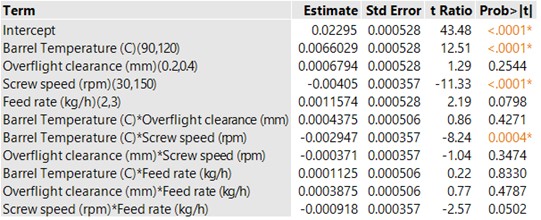 Table 1. Barrel temperature and screw speed has a significant impact on %GABA-L highlighting the importance of local heat in maintaining drug stability (p < 0.05 for statistical significance)
Table 1. Barrel temperature and screw speed has a significant impact on %GABA-L highlighting the importance of local heat in maintaining drug stability (p < 0.05 for statistical significance)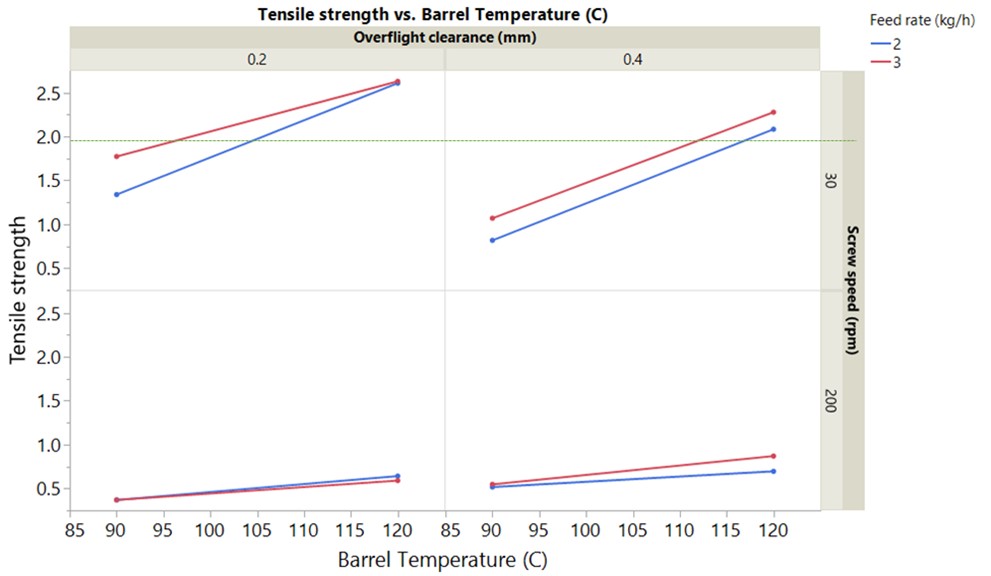 Figure 1. Low screw speed resulted in compressible and strong granules due to higher degree of fill during granulation. (Tensile strength as a function of independent process variables. The granules were compressed using a STYL’one compaction simulator at compaction pressure of 254 MPa.)
Figure 1. Low screw speed resulted in compressible and strong granules due to higher degree of fill during granulation. (Tensile strength as a function of independent process variables. The granules were compressed using a STYL’one compaction simulator at compaction pressure of 254 MPa.)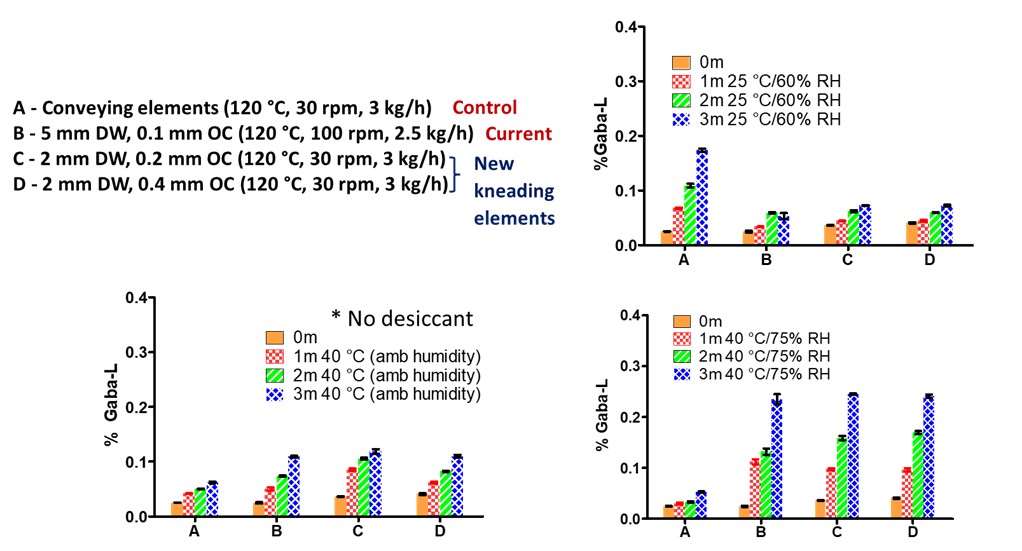 Figure 2. High temperature and high humidity resulted in higher GABA-L content. Storage at 40 °C, ambient humidity points out towards a stable gabapentin product with shelf life of 2 years based on guidelines. (No desiccant was added to any of them to ensure the extreme storage stability case)
Figure 2. High temperature and high humidity resulted in higher GABA-L content. Storage at 40 °C, ambient humidity points out towards a stable gabapentin product with shelf life of 2 years based on guidelines. (No desiccant was added to any of them to ensure the extreme storage stability case)