Formulation and Delivery - Chemical
Category: Poster Abstract
(M1330-02-08) A Single-Step Extrusion Process for Formulation Development of Self-Emulsifying Granules for Oral Delivery of a BCS Class IV Drug
Monday, October 23, 2023
1:30 PM - 2:30 PM ET
- VK
Vineet R. Kulkarni, B.Pharm, M.S. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - VK
Vineet R. Kulkarni, B.Pharm, M.S. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - SB
Santosh Bashyal, Ph.D. (he/him/his)
University of Texas at Austin
Austin, Texas, United States - VN
Varsha V. Nair, B.Pharm, M.S. (she/her/hers)
University of Texas at Austin
Autin, Texas, United States - MM
Mohammed Maniruzzaman, Ph.D., SRPharmS, MAS (he/him/his)
University of Texas at Austin
Austin, Texas, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: The aim of the study was to optimize the formulation development of a BCS Class IV drug by improving the solubility and permeability. The study investigated the utility of self-emulsifying solid lipid matrices as carrier systems for BCS Class IV drugs. Self-emulsifying drug delivery systems (SEDDS) have been extensively investigated for formulating the drug class of interest here, however, manufacturing SEDDS is challenging.1 These systems usually have low drug loading capacities, and the incorporated drugs tend to recrystallize on storage, which severely impacts the storage stability in vitro and performance in vivo.2 Moreover, they require greater amounts ( >80%) of lipid carriers, co-solvents, surfactants, and other excipients to keep them from recrystallizing. This in turn is again challenging for high-dose drugs as it affects the size of the final drug product (tablets and capsules).3 Also, the final liquid nature of the formulation affects the handling and processability of the formulation which possesses challenges during the manufacturing and packaging steps.1–3 In this work, we have studied the feasibility of a single-step extrusion process to formulate and optimize solid self-emulsifying granules with a relatively higher drug loading for Ritonavir (RTV), which is a BCS Class IV drug. Further, we have compared the performance of using these granules as the feedstock for direct powder extrusion-based 3D printing as opposed to the use of physical blends. The stability and solubility-permeability advantage of these granules was also evaluated.
Methods: Ritonavir (RTV) was selected as a BCS Class IV drug model drug. Excipient screening for lipids and surfactants was carried out by evaluation of drug supersaturation levels in lipid matrices, followed by final state analysis of lipid-surfactant mixtures. Phase diagram studies along with particle size analysis were carried out to select a final lipid-surfactant combination from the screened systems. The design of the Experiment (DoE) approach was used to optimize the extrusion process for processing temperature and speed to process granules with optimized attributes. Lipid-based granules obtained during the extrusion process were evaluated for their performance in vitro using non-sink supersaturation studies. Formulated granules and granules loaded capsules underwent dissolution testing using a USP type II apparatus with low volume vessels at 37.0 ± 0.5 °C and a paddle speed of 7 rpm. The pH-shift dissolution tests were conducted in 120 mL of 0.1N HCl for 2h, followed by shift to pH 6.8 by addition of 0.1N phosphate buffer for 2h. Granules processed using extrusion were compared to the conventional process of melt mixing for the preparation of self-emulsifying systems. Solid-state (x-ray diffraction analysis) and thermal analysis (differential scanning calorimetry) were carried out at every stage to understand the effects of different conditions on the solid-state and thermal behavior of the involved drug and excipients in the delivery system. Infrared spectroscopy (FT-IR) was used to confirm the absence of any unwanted chemical interactions between the formulation components and the drug.
Results: The excipients Gelucire G48/16 (G48/16) and a 1:1 mixture of G48/16 and Gelucire G44/14 (G44/14) showed maximum drug supersaturation of 38 and ~39.5 %w/w. These two lipids on screening with a range of surfactants resulted in a solid state of the final formulation with polyethylene glycol (PEG) 3350 and PEG 6000. Evaluation of the combinations of these two lipids and surfactants using phase diagram studies resulted in a broader region for forming clear emulsions post-dispersion for G48/16-PEG3350 system with minimal dispersion times and no observed phase separation. Particle size analysis for different ratios of the same yielded a monodispersed nano-ranged system at a 9:1 ratio of G48/16-PEG3350. This carrier system with a 40% drug loading was selected to formulate granules. Processed granules showed approximately 27 times increase in apparent solubility when tested in deionized water and a maximum 103-fold increase in apparent solubility, later maintained at approximately 87 folds when tested using a pH-shift system.
Conclusion: In this study, we have developed self-emulsifying granules using a solvent-free, lipid carrier-mediated, hot-melt extrusion process. The drug-loaded granules significantly increased the solubility and achieved the target release with an 87-fold increase in apparent solubility for RTV, a BCS Class IV drug with a 40% drug load. We were also able to improve the post-handling of SEDDS by the use of high melting point lipid carriers. Solid self-emulsifying lipid carriers are a more stable and practical alternative dosage form for BCS Class IV drugs, especially for drugs with higher doses, as these can load more drugs due to the immediate phase transition nature of the carriers and also have better stability in storage due to higher melting points.
References: 1. Aloisio C, Shah A V., Longhi M, Serajuddin ATM. Development of self-microemulsifying lipid-based formulations of trans-resveratrol by systematically constructing lipid-surfactant-water phase diagrams using long-chain lipids. Drug Dev Ind Pharm. 2021;47(6):897-907. doi:10.1080/03639045.2021.1934866
2. Friedl JD, Jörgensen AM, Le‐Vinh B, Braun DE, Tribus M, Bernkop-Schnürch A. Solidification of self-emulsifying drug delivery systems (SEDDS): Impact on storage stability of a therapeutic protein. J Colloid Interface Sci. 2021;584:684-697. doi:10.1016/j.jcis.2020.11.051
3. Silva LAD, Almeida SL, Alonso ECP, et al. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. Int J Pharm. 2018;541(1-2):1-10. doi:10.1016/j.ijpharm.2018.02.020
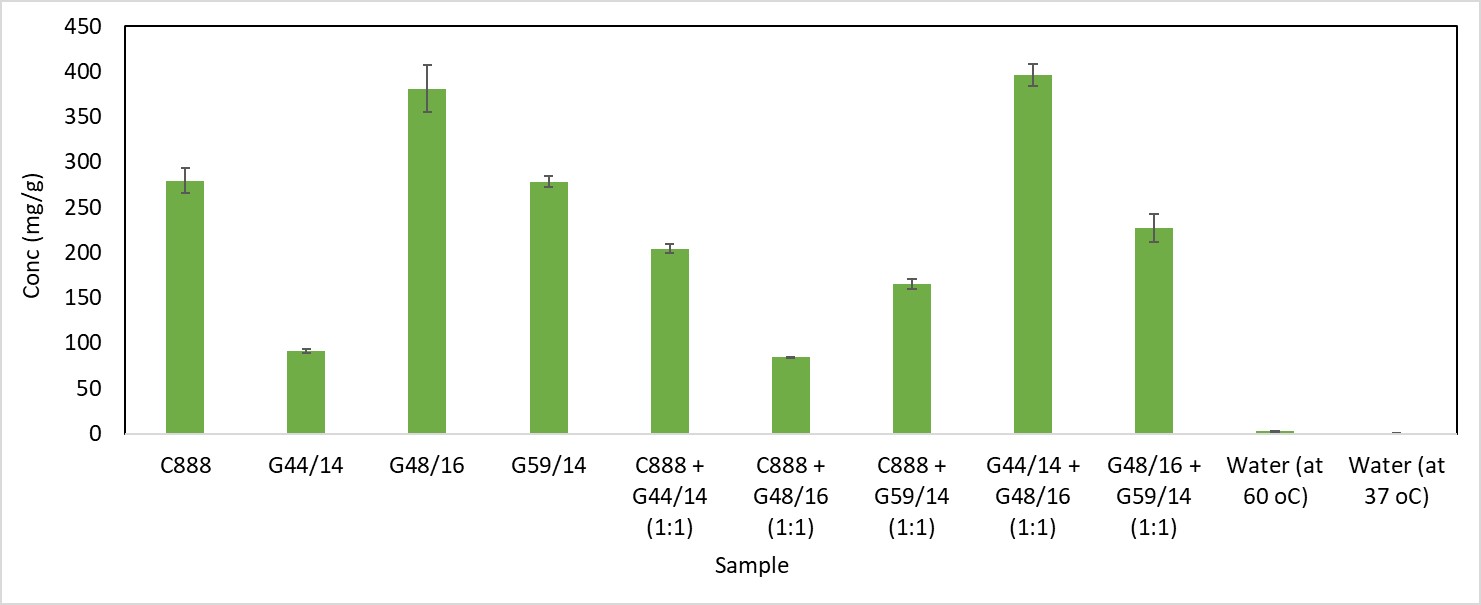 Supersaturation solubility screening for ritonavir (RTV) across different lipids and surfactant combinations. The samples were run in triplicates and the data mentioned as average +/- standard deviation. The G48/16 and G44/14 + G59/14 showed improved drug loading compared to other groups.
Supersaturation solubility screening for ritonavir (RTV) across different lipids and surfactant combinations. The samples were run in triplicates and the data mentioned as average +/- standard deviation. The G48/16 and G44/14 + G59/14 showed improved drug loading compared to other groups.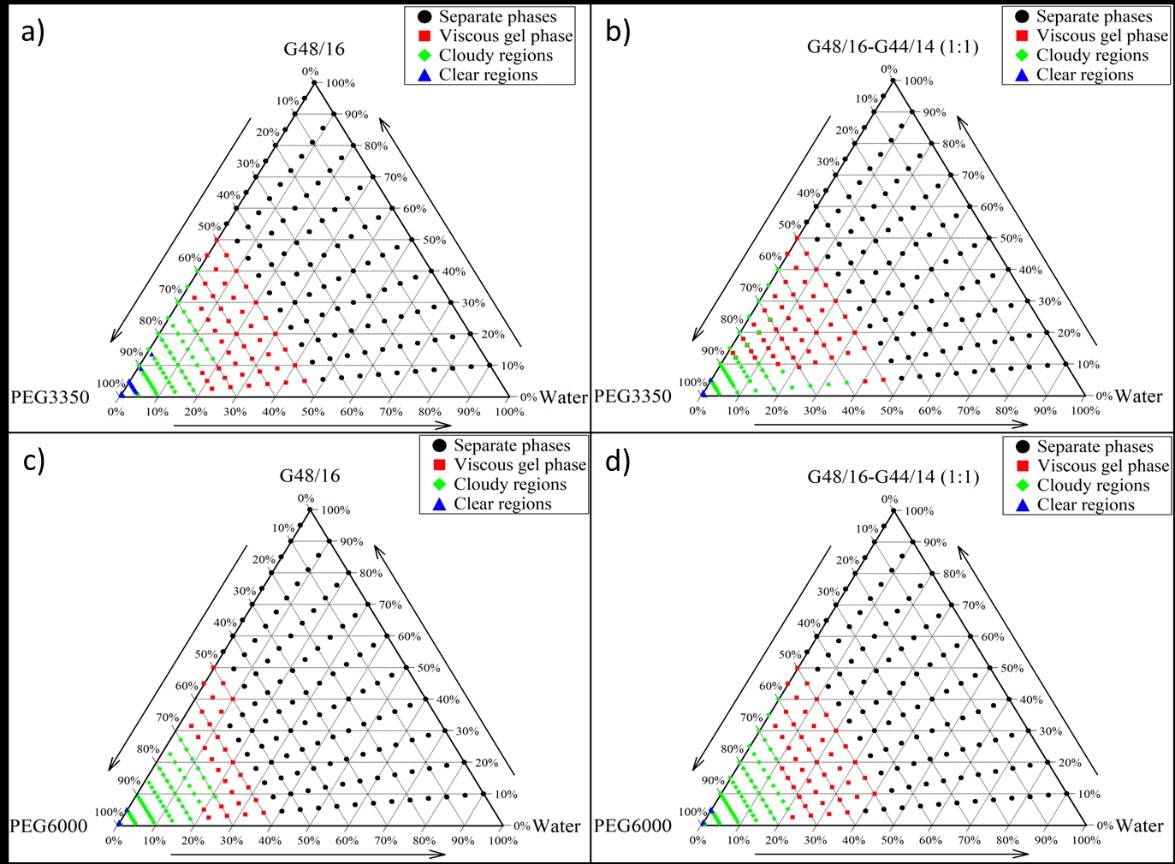 Pseudo-ternary and ternary phase diagram for a) G48/16 - PEG3350 - Water, b) (G48/16 + G44/14) - PEG3350 – Water, c) G48/16 – PEG6000 – Water, d) (G48/16 + G44/14) – PEG6000 – Water mixtures. The samples were run in triplicates.
Pseudo-ternary and ternary phase diagram for a) G48/16 - PEG3350 - Water, b) (G48/16 + G44/14) - PEG3350 – Water, c) G48/16 – PEG6000 – Water, d) (G48/16 + G44/14) – PEG6000 – Water mixtures. The samples were run in triplicates..jpg) In vitro, non-sink dissolution testing of pure drug, ritonavir (RTV), and optimized formulated drug-loaded granules.
In vitro, non-sink dissolution testing of pure drug, ritonavir (RTV), and optimized formulated drug-loaded granules. 