Manufacturing and Analytical Characterization - Chemical
Category: Poster Abstract
(T1230-08-55) Long-Term Stability of In-House Sunscreen Formulations

Yang Yang, PhD (she/her/hers)
Research Staff Fellow
US Food and Drug Administration
Silver Spring, Maryland, United States- AW
Apipa Wanasathop, Ph.D. (she/her/hers)
US Food and Drug Administration
Silver Spring, Maryland, United States - KR
Kartik Roy, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - AA
Ann-marie Afrifa, Ph.D. (she/her/hers)
US Food and Drug Administration
Silver Spring, Maryland, United States - JW
Jiang Wang, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - SC
Sergio Coelho, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - SA
Steven Adah, Ph.D. (he/him/his)
US Food and Drug Administration
SILVER SPRING, Maryland, United States - PF
Patrick Faustino, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - MA
Muhammad Ashraf, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States - XX
Xiaoming Xu, Ph.D.
US Food and Drug Administration
Silver Spring, Maryland, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Sunscreens are usually formulated as oil-in-water emulsions (cream or lotions). In this system, an oil phase is evenly dispersed as globules in a continuous aqueous phase. UV filters are the active ingredients in sunscreen, which protect the skin by absorbing, reflecting, or scattering UV radiation at the skin surface. It has been shown that certain sunscreen active ingredients may be dermally absorbed resulting in systemic exposure. There are several factors that may affect skin absorption including physical characteristics and composition of the sunscreen formulation. The objective of this study was to investigate the physical and chemical stability of in-house prepared oil-in-water sunscreen formulations that were stored at 25°C/60% RH for one-year.
Methods: A series of in-house sunscreen products were prepared by varying manufacturing process parameters including homogenization speed and temperature. The sunscreen products, containing 3% (w/w) avobenzone, 10% (w/w) octocrylene, and 6% (w/w) oxybenzone as active ingredients, were prepared and stored at 25°C/60% RH. The physical stability of the sunscreen products was determined by measuring the globule size distribution, viscosity, pH and visual appearance. The chemical stability of the sunscreens was determined by measuring and comparing the active ingredients and impurity levels in fresh and old sunscreen products using a validated and stability indicating UPLC-UV method (Fig.1) [Yang Y. et. al. Journal of Investigative Dermatology 2020]. To evaluate the skin permeation of the chemical UV filters, in vitro skin permeation tests (IVPT) were performed using excised human skin (New York Firefighters Skin Bank, NY, USA) mounted on in-line flow through diffusion cells (PermeGear, Inc., PA, USA). PBS containing 4% (w/v) of bovine serum albumin (BSA) was used as the receptor solution and was delivered through the in-line diffusion cells at a flow rate of 25 µL/min. IVPT samples were collected at predetermined time points for 24 hours.
Results: No significant changes were found in the visual appearance (cream color, phase separation), pH, and globule size distribution of the in-house sunscreen products after one year of storage as compared to fresh sunscreen samples. All three UV filters remained chemically stable with assay (90-110%) and impurity levels (NMT 3%) within specification. However, the yield stress and viscosity at medium shear rate significantly increased in the one-year stability samples (p < 0.05, Fig. 2) as compared to the fresh sunscreen samples. Furthermore, oxybenzone exhibited a higher skin permeation from the one-year stability samples than from the fresh sunscreen samples (p < 0.05, Fig. 3). On the other hand, avobenzone and octocrylene were detected in the receptor solution but below the lowest limit of quantification (less than 0.5 µg/mL).
Conclusion: After long-term storage (25°C/60% RH) for one year, the in-house sunscreen products demonstrated changes in yield stress and viscosity, which lead to significant increase in the skin permeation of oxybenzone. These preliminary results suggest that the stability of the product may lead to differences in the skin absorption of UV filters.
References: 1. Yang Yang, et. al., "In Vitro Testing of Sunscreens for Dermal Absorption: Method Comparison and Rank Order Correlation with In Vivo Absorption" AAPS PharmSciTech . 2022 Apr 22;23(5):121. doi: 10.1208/s12249-022-02275-z.
2. Yang Yang, et. al., "In Vitro Testing of Sunscreens for Dermal Absorption: A Platform for Product Selection for Maximal Usage Clinical Trials" Journal of Investigative Dermatology Volume 140, Issue 12, December 2020, Pages 2487-2495
Acknowledgements: Disclaimer: This poster reflects the views of the authors and should not be construed to represent the views or policies of the Food and Drug Administration. Funding support: FDA intramural research fund, which provides ORISE fellowships to postdoctoral fellows.
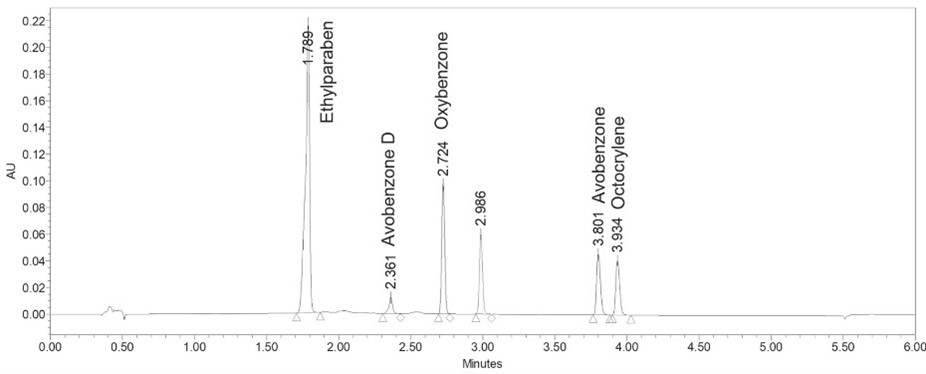 Figure 1. UPLC Chromatograms of chemical UV filters and its impurity (avobenzone D).
Figure 1. UPLC Chromatograms of chemical UV filters and its impurity (avobenzone D).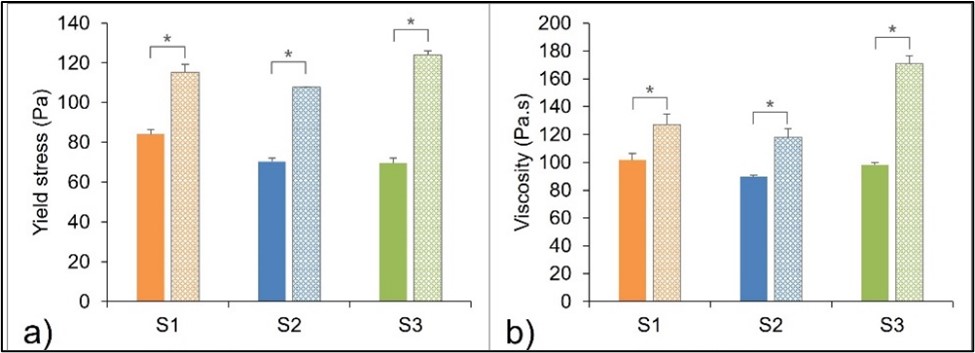 Figure 2. Comparison of (a) yield stress and (b) viscosity at medium shear rate (1 s-1) between time 0 (solid) and after one year storage (dotted), mean ± SD, n = 3, paired t-test (*p < 0.05).
Figure 2. Comparison of (a) yield stress and (b) viscosity at medium shear rate (1 s-1) between time 0 (solid) and after one year storage (dotted), mean ± SD, n = 3, paired t-test (*p < 0.05).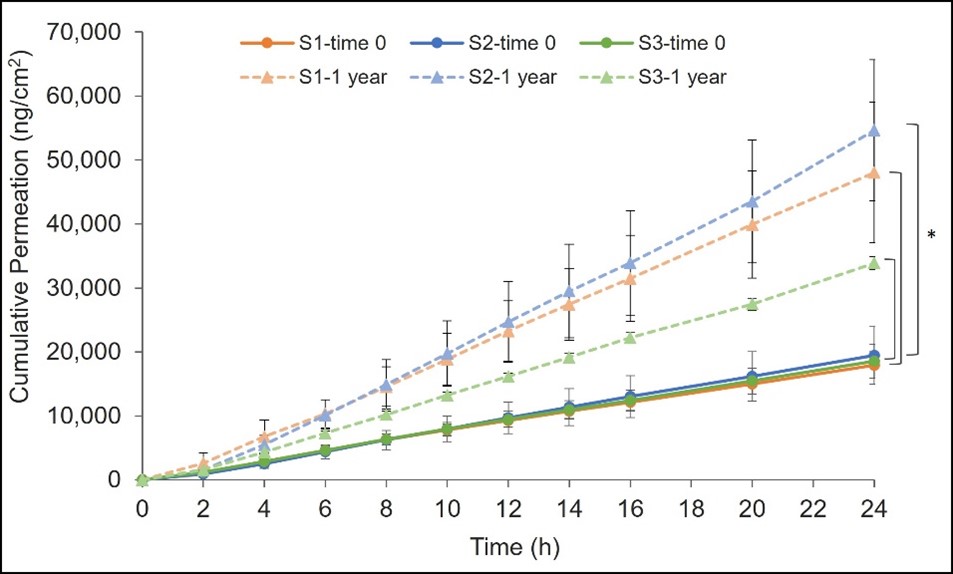 Figure 3. Cumulative permeation of oxybenzone after dose normalizing from time 0 (solid) and after one year of storage (dotted). S1, S2, and S3 representing in orange, blue, and green, respectively. Data expressed as mean (ng/cm2) ± SD, n=3-6, ANOVA (*p < 0.05).
Figure 3. Cumulative permeation of oxybenzone after dose normalizing from time 0 (solid) and after one year of storage (dotted). S1, S2, and S3 representing in orange, blue, and green, respectively. Data expressed as mean (ng/cm2) ± SD, n=3-6, ANOVA (*p < 0.05).