Formulation and Delivery - Chemical
Category: Poster Abstract
(T1230-02-10) Evaluating the Dissolution of Commercially Available Metered Dose Inhaler (MDI) Drug Products from Realistic In Vitro Experiments

Abhinav Mohan, PhD
Research Scientist
US Food and Drug Administration
Silver Spring, Maryland, United States
Abhinav Mohan, PhD
Research Scientist
US Food and Drug Administration
Silver Spring, Maryland, United States- SB
Simon Berger, Ph.D.
University of Florida
Gainesville, Florida, United States - SD
Sneha Dhapare, Ph.D. (she/her/hers)
US Food and Drug Administration
Silver Spring, Maryland, United States - BN
Bryan Newman, Ph.D. (he/him/his)
US Food and Drug Administration
Silver Spring, Maryland, United States - MS
Marten Svensson, Ph.D.
Emmace Consulting
Lund, Skane Lan, Sweden - PE
Peter Elfman, MS
Emmace Consulting
Lund, Skane Lan, Sweden - LW
Lawrence Winner, Ph.D.
University of Florida
Gainesville, Florida, United States - JB
Jürgen B. Bulitta, Ph.D.
University of Florida
Orlando, Florida, United States - GH
Guenther Hochhaus, Ph.D.
University of Florida
Gainesville, Florida, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Currently, regulatory guidelines for quality testing of orally inhaled drug formulations do not include dissolution testing; however, multiple studies have demonstrated a strong relationship between release rate and pharmacokinetic and pharmacodynamic properties [1]. Therefore, to better understand the critical dissolution study parameters that may provide an improved correlation with in vivo performance metrics, this work examined (1) whether achieving a certain drug substance solubility is more critical than the choice of the surfactant when selecting the dissolution media for dissolution experiments, and (2), relevant for sample preparation, how passage through an anatomical mouth-throat (MT) may influence dissolution performance, using commercial MDI products.
Methods: Two commercially available suspension MDI products (Flovent® HFA and Advair® HFA) containing fluticasone propionate (FP) in the United States were utilized for this study. For Aim 1, an abbreviated Andersen Cascade Impactor (aACI), operating at 28.3 L/min, was used to collect the fine particle dose < 3.3 µm (when equipped with the United States Pharmacopeia (USP) throat) or the lung dose (ex-medium Oropharyngeal Consortium (OPC) M mouth throat (MT) model in combination with stage 2) on a filter membrane. After transfer of the filter paper, dissolution studies for FP were conducted using an adapted USP V Apparatus (paddle over disk) with the filter side down in a sandwich orientation [2]. Bovine serum albumin (BSA), Tween-80, and sodium dodecyl sulfate (SDS), dissolved in PBS to achieve the same solubility for the analyte (5 µg/ml) were used as dissolution media at 37oC. After defined time points samples were removed and analyzed for FP content. To determine the effect of MT models on dissolution (Aim 2), the lung dose (ex-throat dose) was collected for a range of MT models utilizing the medium inhalation profile followed by dissolution assessment as described above.
Results: The solubility of FP increased with increasing concentrations of Tween-80 and SDS, with the latter showing the most pronounced solubilization characteristics (Figure 1 A,B). However, it appears there was no relationship between the concentration of BSA and the solubility of FP. To evaluate surfactant impact on MDI’s dissolution profiles at a given solubility, concentrations of SDS and Tween-80 that yielded an FP solubility of 5 µg/ml was studied to understand the dissolution rate of FP from Advair® HFA and Flovent® HFA, respectively (Figure 1 C-F). Using Tween-80 as the surfactant, it was found that FP from Advair® HFA dissolved faster. Comparison of the profiles suggested that the best balance between observed variability and differences between the two formulations was observed for 0.5% Tween-80 providing a FP solubility of 5 µg/ml (Figure 1 A,B). Results agree with literature [3], and with the hypothesis that dissolution rate is only affected by solubility and not the nature of the surfactant. However, given the limited solubility of FP in BSA, dissolution studies with Flovent® HFA and Advair® HFA using BSA were incomplete with only a fraction dissolving over the study duration. When comparing the dissolution of the lung fraction of Advair® HFA and Flovent® HFA to the fine particle fraction (FPF) < 3.3 µm, the Advair® HFA showed a higher rate of dissolution for both the lung fraction and fine particle fraction < 3.3 µm. However, for both drug products, the material collected as lung dose (using the OPC M MT model to collect material passing stage 1) appeared to dissolve faster than the FPF < 3.3 µm (USP throat, material passing stage 2 of the aACI; Figure 2). Whether this is related to the OPC M throat or the USP throat needs further investigation, especially as our results would otherwise contradict the general understanding that smaller particles depositing on higher stage numbers will dissolve faster [3,4]. The choice of the MT model appeared to influence dissolution of FP from Flovent® HFA at earlier time points (Figure 3). The OPC S MT appeared to facilitate a faster dissolution, passage through which may have occurred quicker as compared to the other MT models which showed comparable dissolution profiles.
Conclusion: The use of synthetic surfactants in this research indicated that solubility may be more critical for impacting dissolution performance as compared to the choice of surfactant used in the study design. Both SDS and Tween-80 enabled solubilities of fluticasone propionate that allowed its dissolution to be effectively studied, which was not observed with BSA. Therefore, the results from dissolution studies were not sufficient to evaluate whether a more bio-relevant medium like BSA also suggest that drug substance solubility is the critical study design factor over surfactant choice. Further studies are warranted regarding surfactant selection with bio-relevant mediums and drug substance solubility to better understand their impact on dissolution performance. Furthermore, it appeared that the choice of the MT model affected the dissolution of FP, as observed from the Flovent® HFA study, indicating the dissolution study outcomes may be sensitive to choice of the MT model.
References: 1. Amini, E., & Hochhaus, G. (2021). Dissolution and drug release. In Inhaled Medicines (pp. 225-266). Academic Press.
2. Hochhaus, G., Chen, M. J., Kurumaddali, A., Schilling, U., Jiao, Y., Drescher, S. K., ... & Bulitta, J. B. (2021). Can pharmacokinetic studies assess the pulmonary fate of dry powder inhaler formulations of fluticasone propionate?. The AAPS Journal, 23, 1-14.
3. Amini, E., Kurumaddali, A., Bhagwat, S., Berger, S. M., & Hochhaus, G. (2021). Optimization of the Transwell® system for assessing the dissolution behavior of orally inhaled drug products through in vitro and in silico approaches. Pharmaceutics, 13(8), 1109.
4. Hartung, N., & Borghardt, J. M. (2020). A mechanistic framework for a priori pharmacokinetic predictions of orally inhaled drugs. PLoS Computational Biology, 16(12), e1008466.
Acknowledgements:Funding for this work was made possible, in part, by the U.S. Food and Drug Administration through Contract 75F40119C10154 and Grant U01FD004943. Abhinav Mohan was supported by a fellowship program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and the U.S. FDA. The views expressed in this abstract are from the authors only and do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
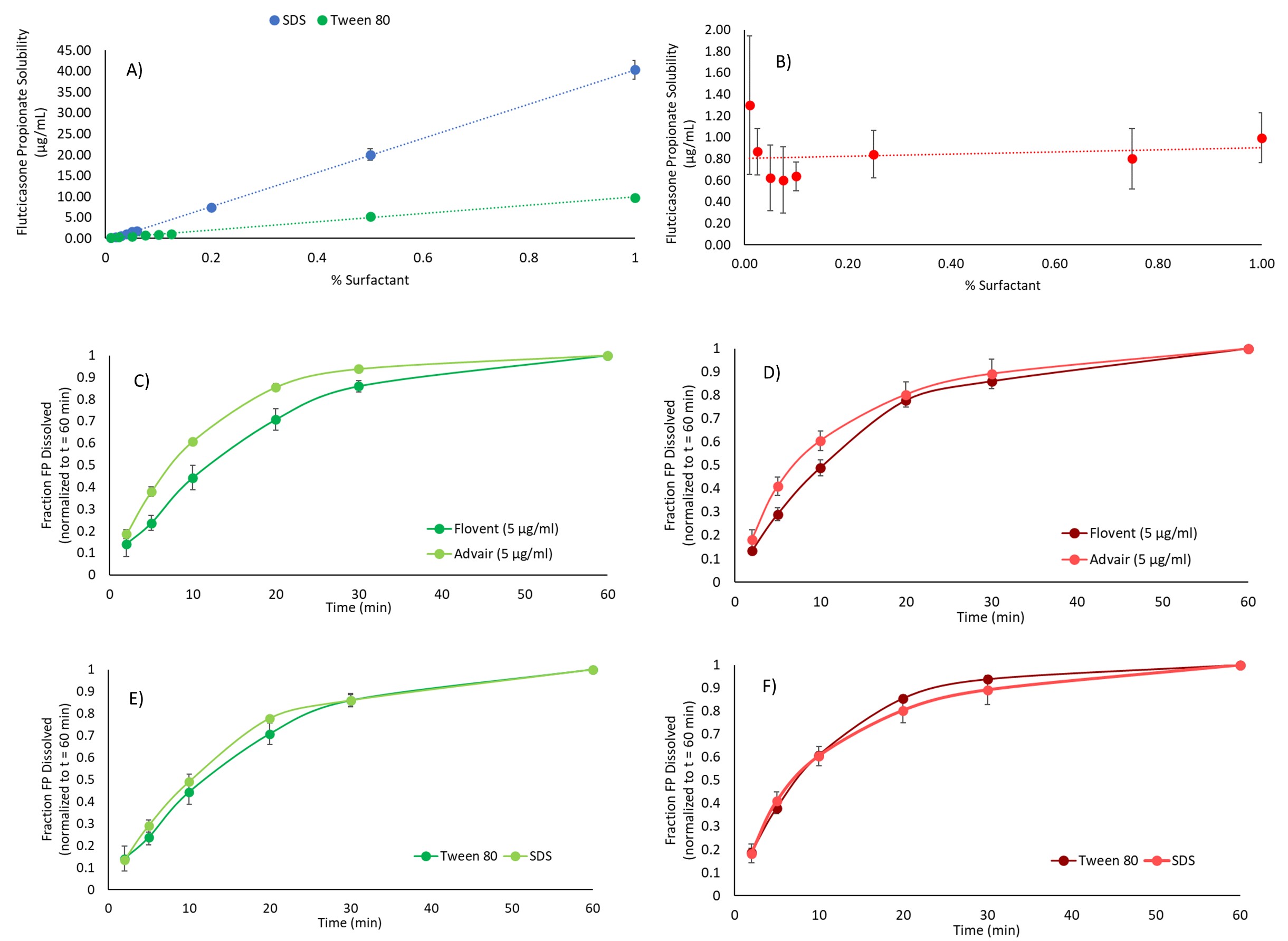 Figure 1: Solubilities of fluticasone propionate API in varying concentrations of A) SDS, Tween-80 and B) BSA, dissolution of Fluticasone Propionate from Flovent® HFA and Advair® HFA at a solubility of 5 µg/ml in C) Tween-80 and D) SDS, and dissolution of Fluticasone Propionate from E) Flovent® HFA and F) Advair® HFA in Tween-80 and SDS at a solubility of 5 µg/ml. Data and error bars: mean ± SD of N = 3 – 5 per datapoint.
Figure 1: Solubilities of fluticasone propionate API in varying concentrations of A) SDS, Tween-80 and B) BSA, dissolution of Fluticasone Propionate from Flovent® HFA and Advair® HFA at a solubility of 5 µg/ml in C) Tween-80 and D) SDS, and dissolution of Fluticasone Propionate from E) Flovent® HFA and F) Advair® HFA in Tween-80 and SDS at a solubility of 5 µg/ml. Data and error bars: mean ± SD of N = 3 – 5 per datapoint.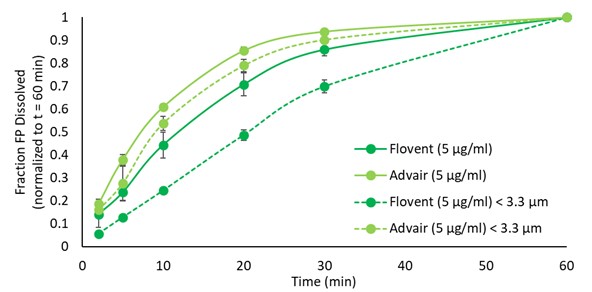 Figure 2: Effect of differences in particle size on dissolution profiles of Fluticasone Propionate from Flovent® HFA and Advair® HFA in Tween-80 at a solubility of 5 µg/ml. Data and error bars: mean ± SD of N = 3 per datapoint.
Figure 2: Effect of differences in particle size on dissolution profiles of Fluticasone Propionate from Flovent® HFA and Advair® HFA in Tween-80 at a solubility of 5 µg/ml. Data and error bars: mean ± SD of N = 3 per datapoint.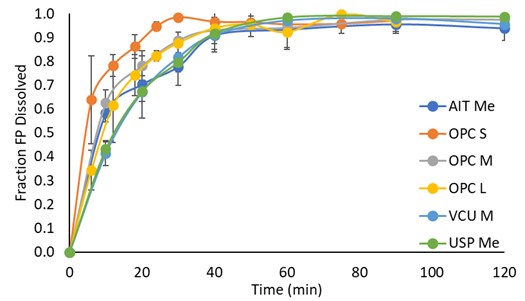 Figure 3: In vitro dissolution profiles for different MT models and medium inhalation profile for Flovent® HFA. AIT = Alberta Idealized Throat; OPC = Oropharyngeal Consortium; VCU = Virginia Commonwealth University; USP = United States Pharmacopeia. Data and error bars: mean ± SD of N = 3 per datapoint.
Figure 3: In vitro dissolution profiles for different MT models and medium inhalation profile for Flovent® HFA. AIT = Alberta Idealized Throat; OPC = Oropharyngeal Consortium; VCU = Virginia Commonwealth University; USP = United States Pharmacopeia. Data and error bars: mean ± SD of N = 3 per datapoint.